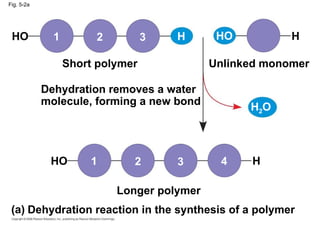
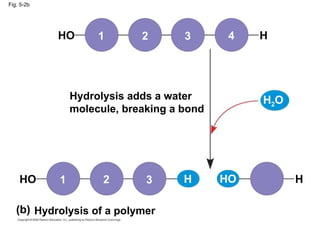
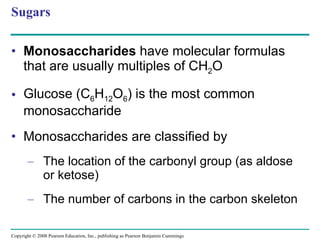
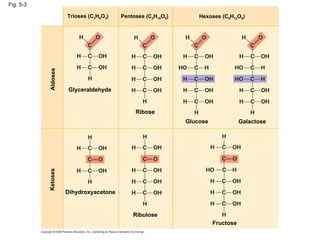
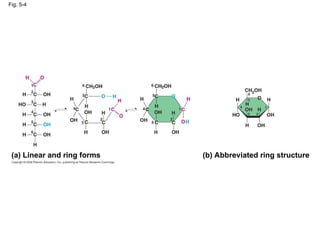
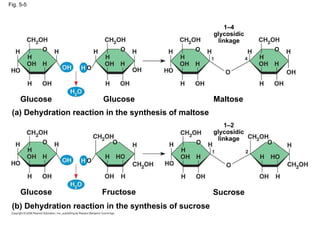
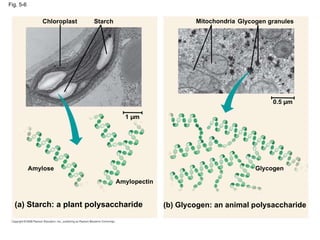
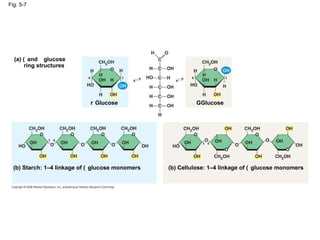
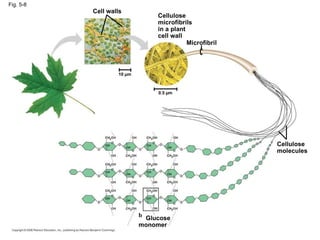
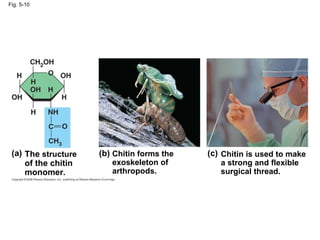
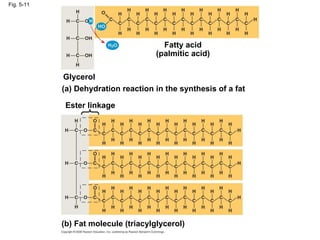
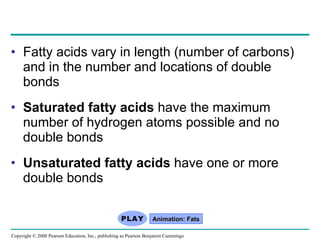
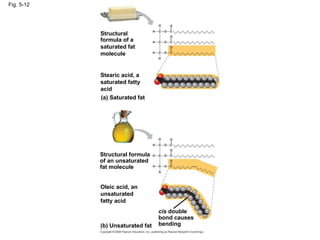
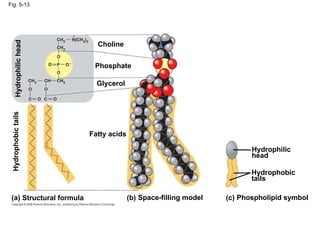
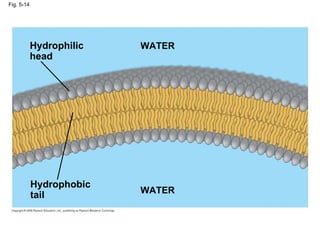
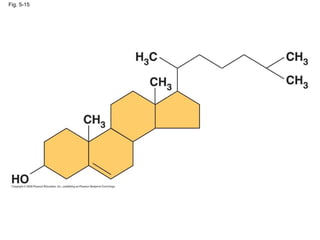
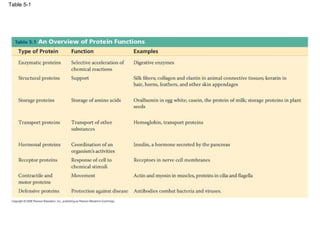
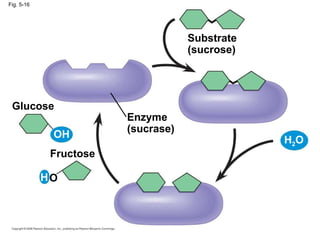
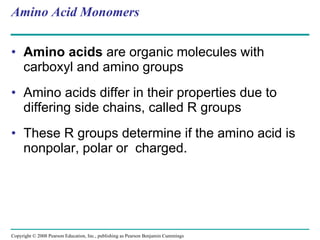
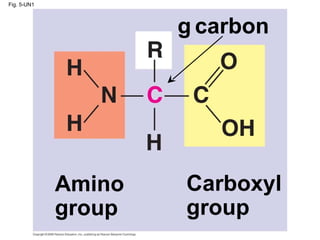
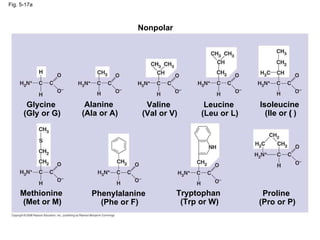
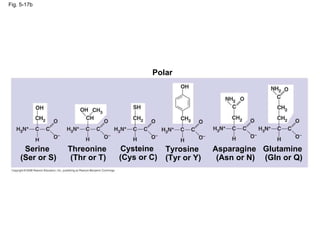
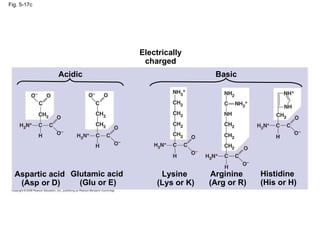
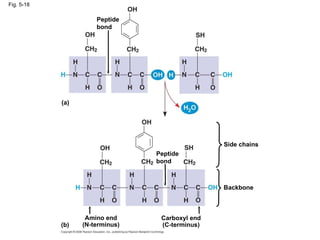
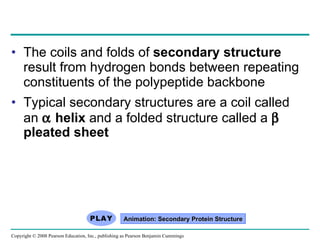
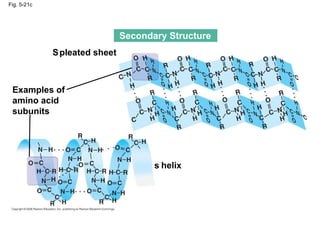
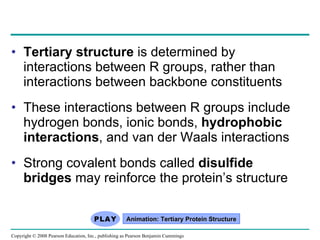
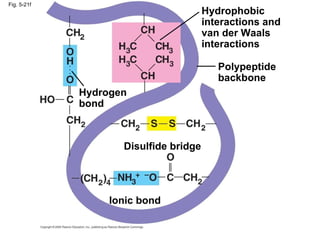
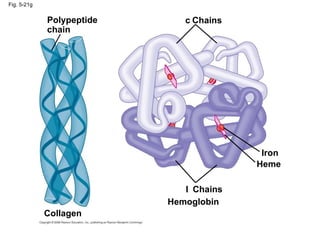
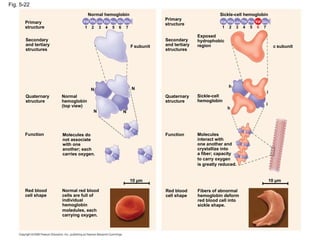
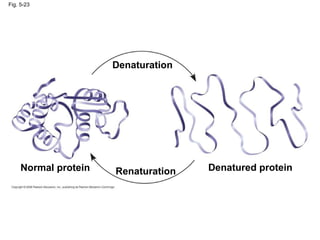
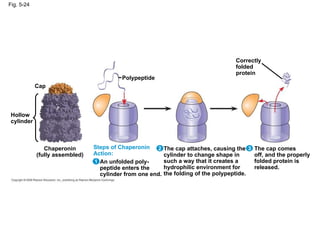
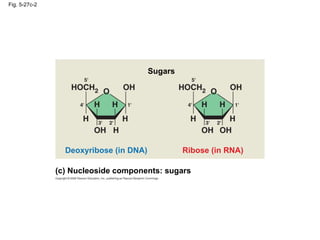
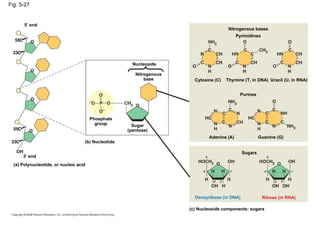
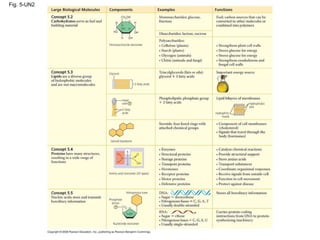
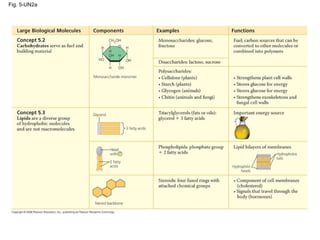
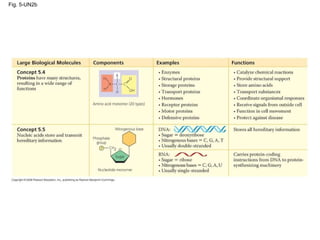
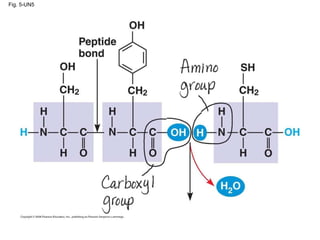
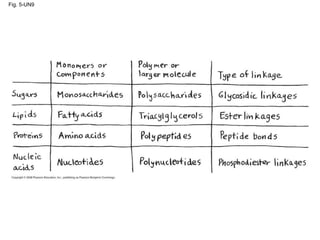
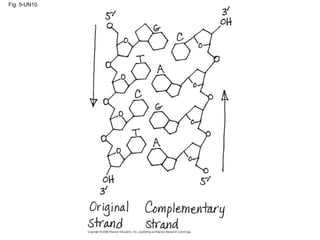
The document summarizes key concepts about macromolecules. It discusses that polymers are composed of monomers linked by dehydration reactions, and examples include carbohydrates, proteins, and nucleic acids. It also explains that lipids are not polymers and include fats, phospholipids, and steroids. Proteins have complex structures including primary, secondary, tertiary and quaternary levels that determine their functions.