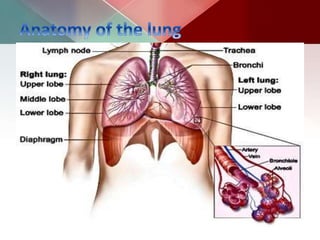
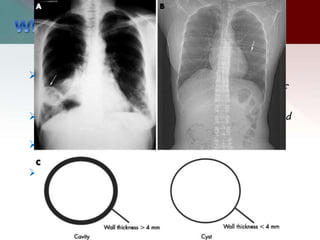
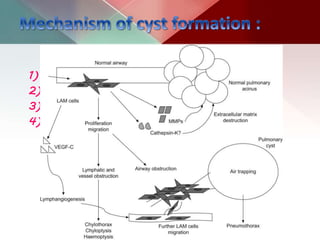
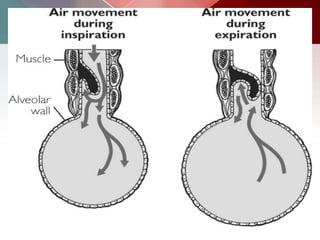
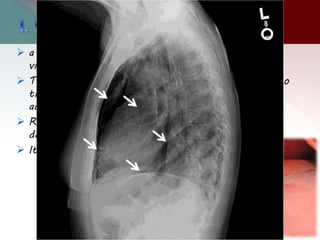
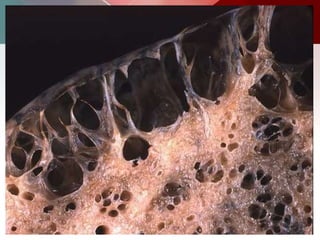
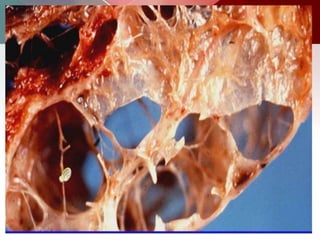
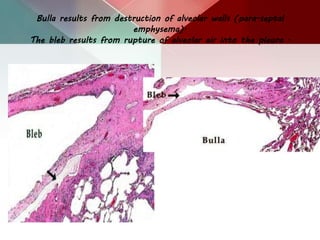
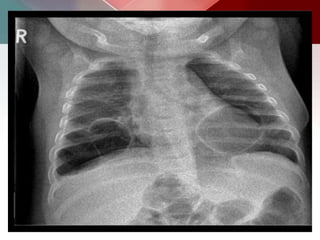
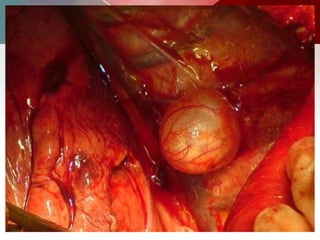
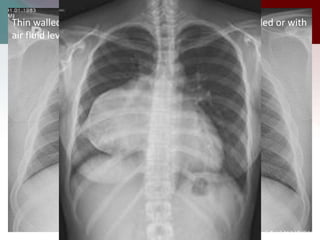
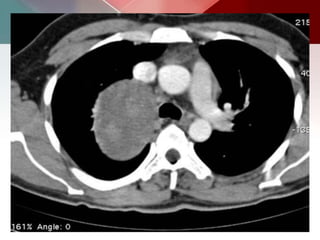
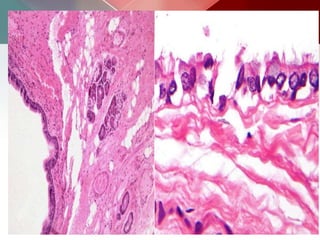
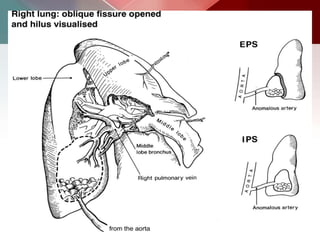
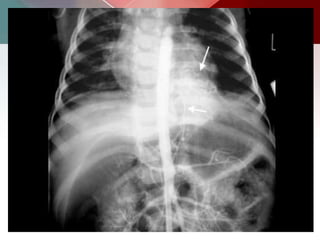
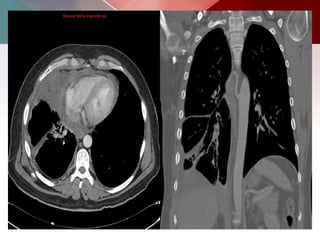
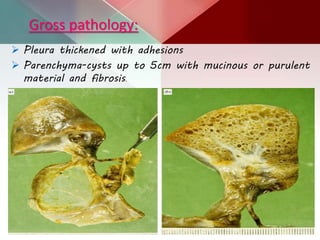
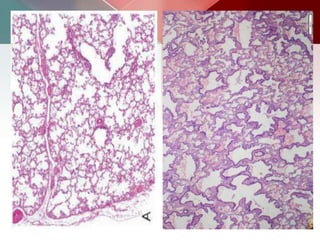
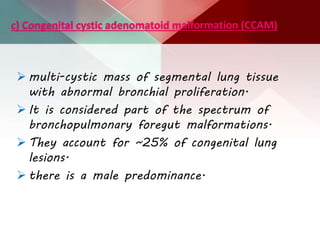
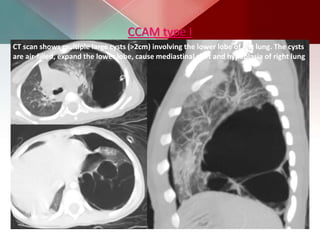
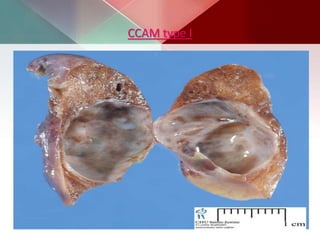
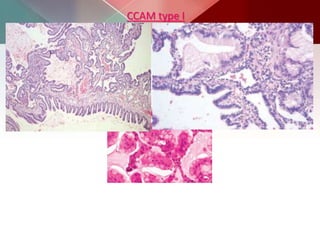
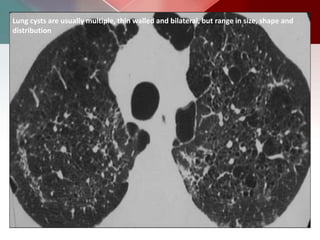
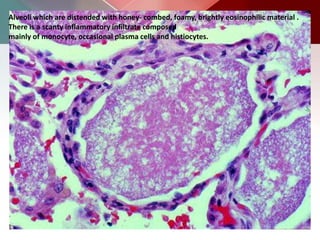
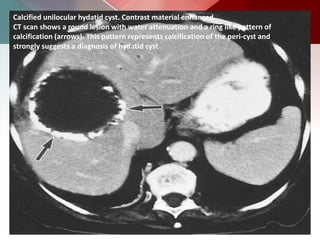
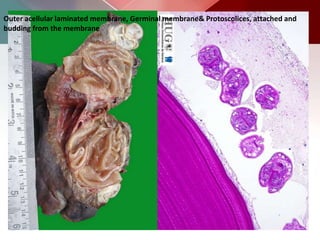
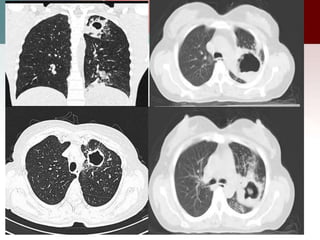
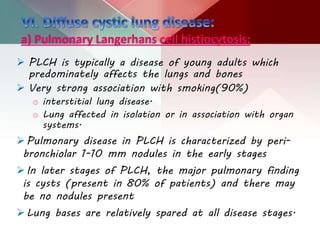
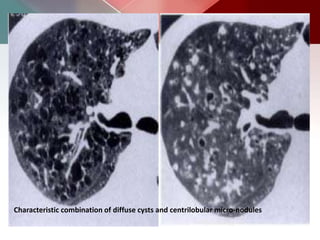
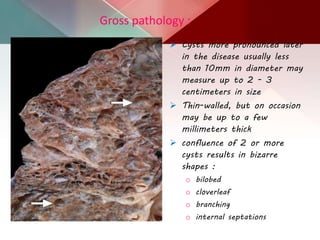
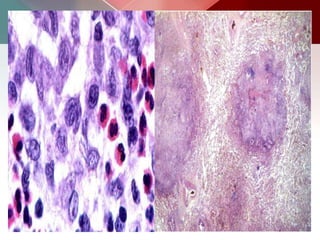
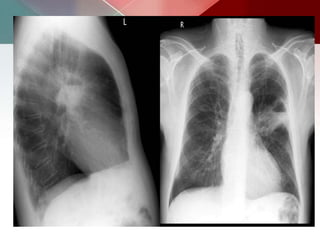
This document describes different types of lung cysts and cavities seen on imaging. It discusses the definitions, causes, and key radiologic features of pulmonary cysts, cavities, blebs, bullae, pneumatoceles, and other cystic lung lesions. Specific entities covered in detail include bronchogenic cysts, pulmonary sequestration, congenital cystic adenomatoid malformation, Langerhans cell histiocytosis, hydatid cysts, and cavitating tuberculosis. The document provides useful information to help distinguish these various cystic lung conditions based on appearance, characteristics, and associated clinical findings.