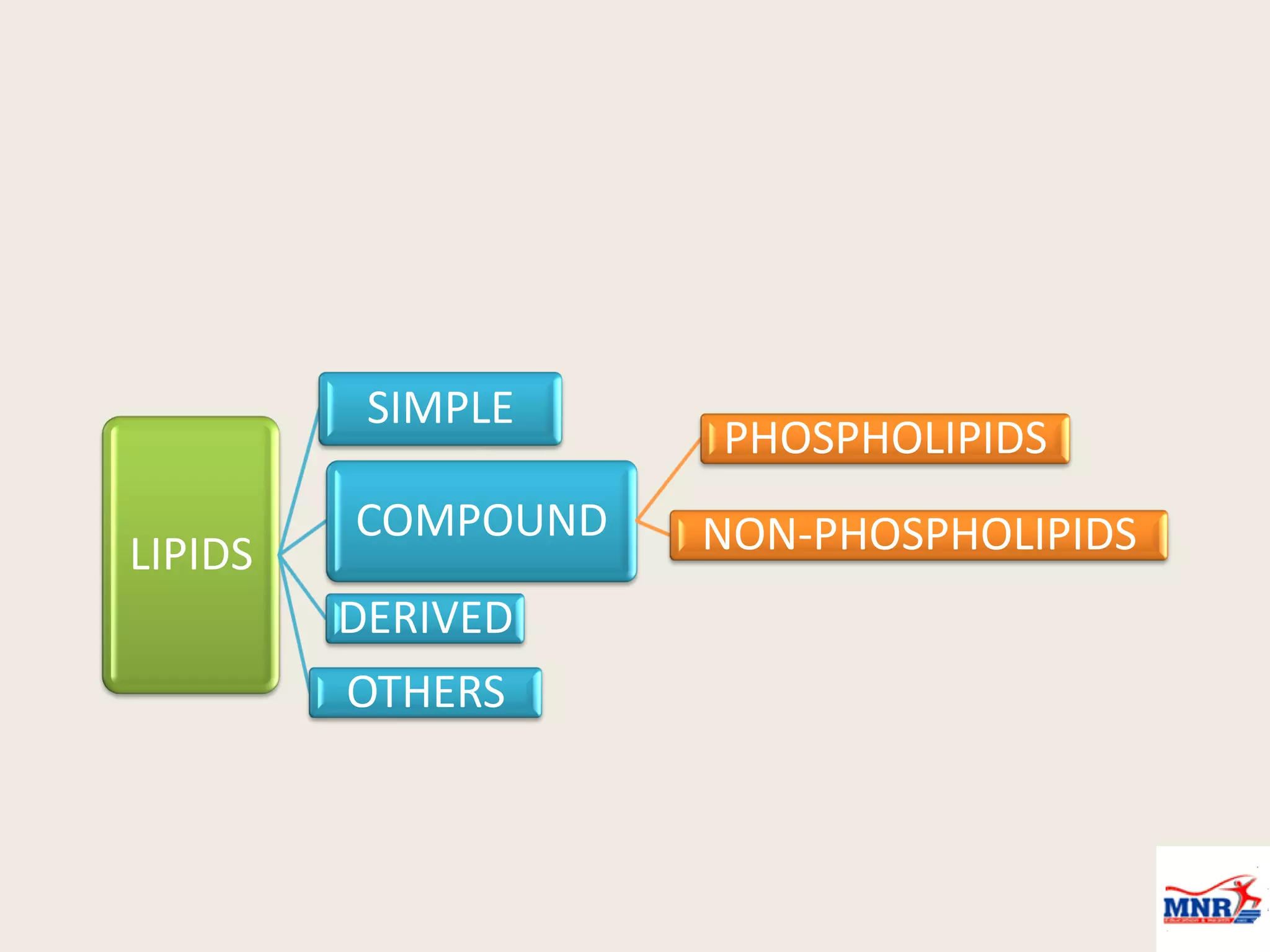
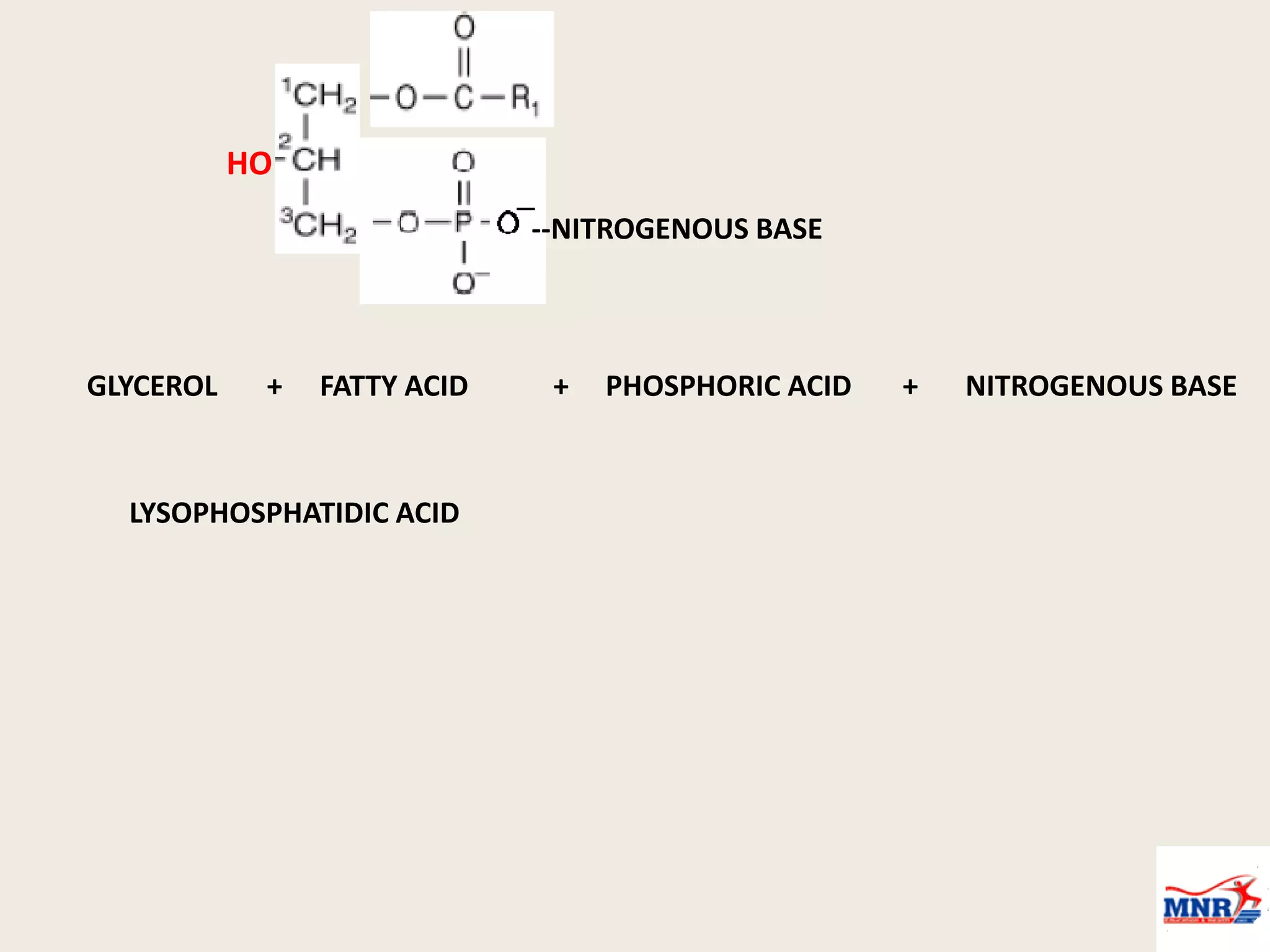
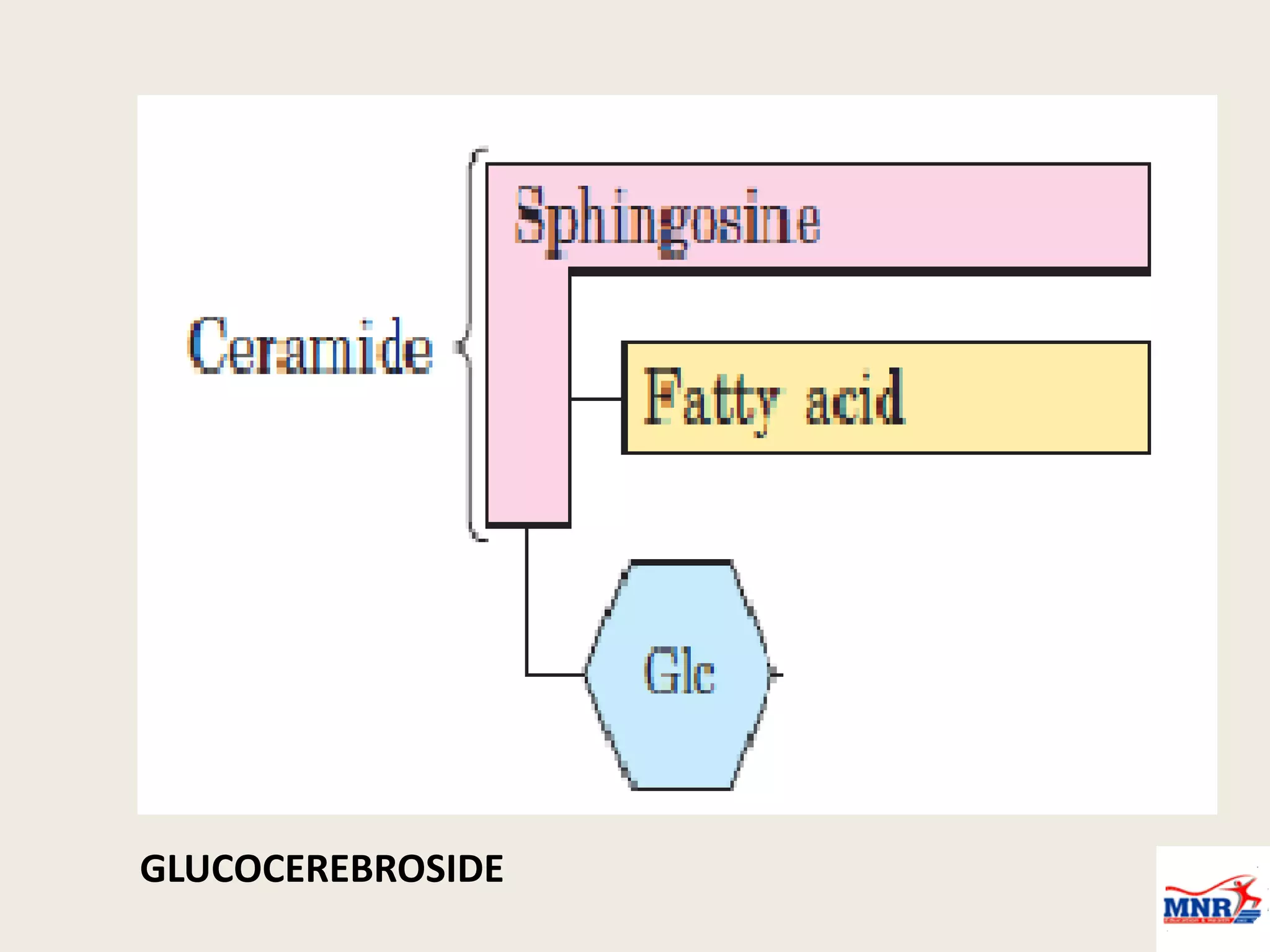
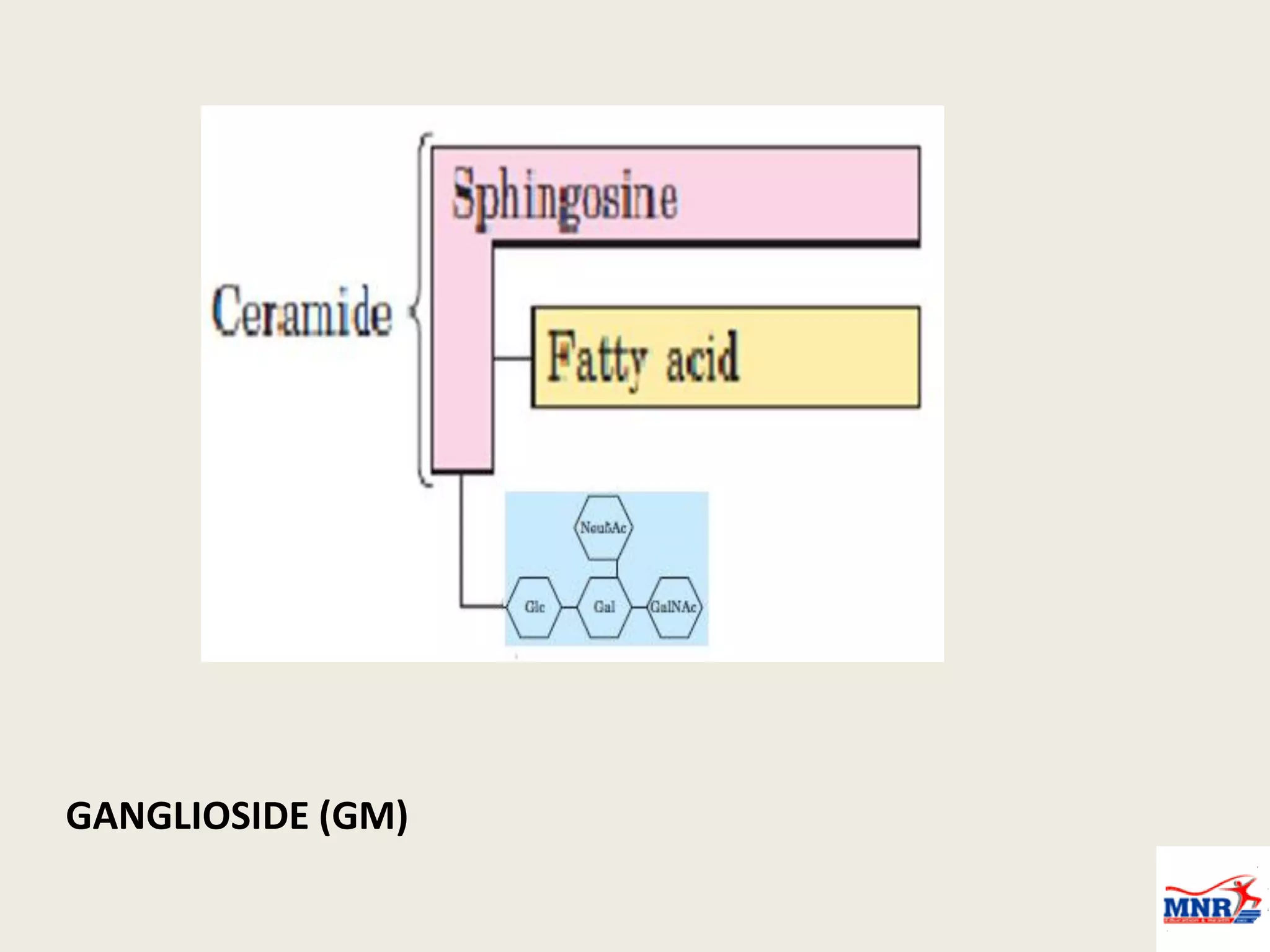
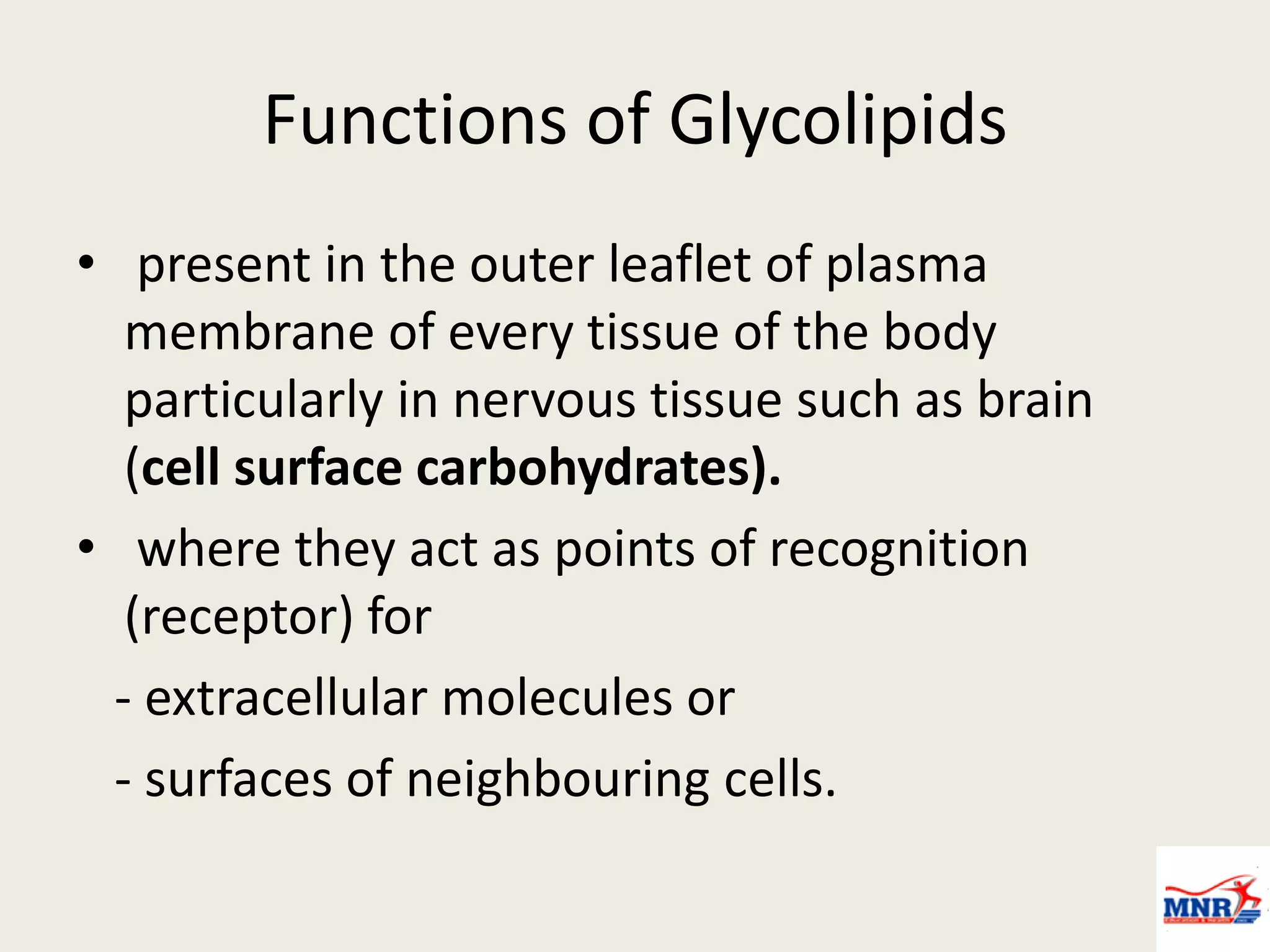
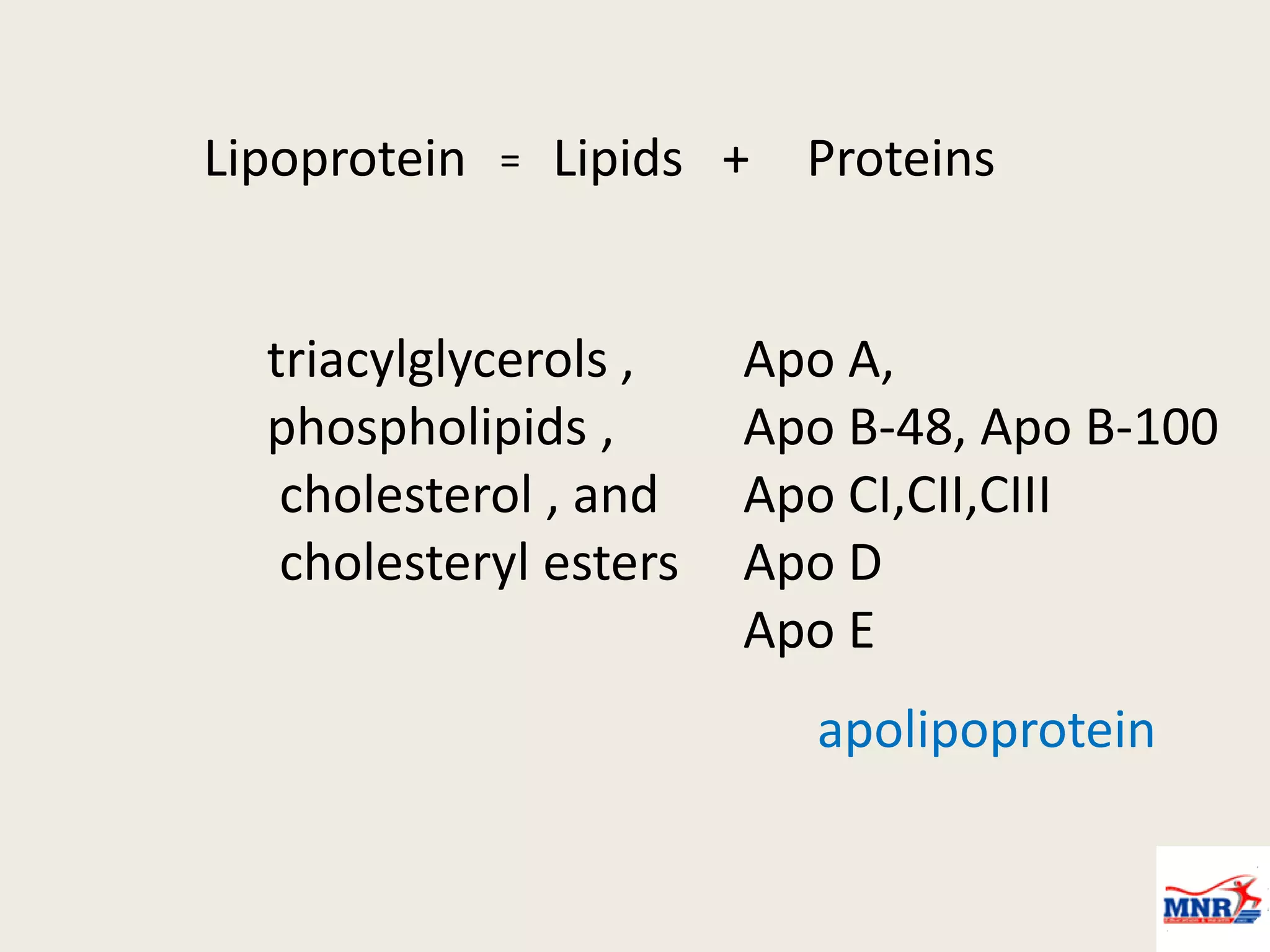
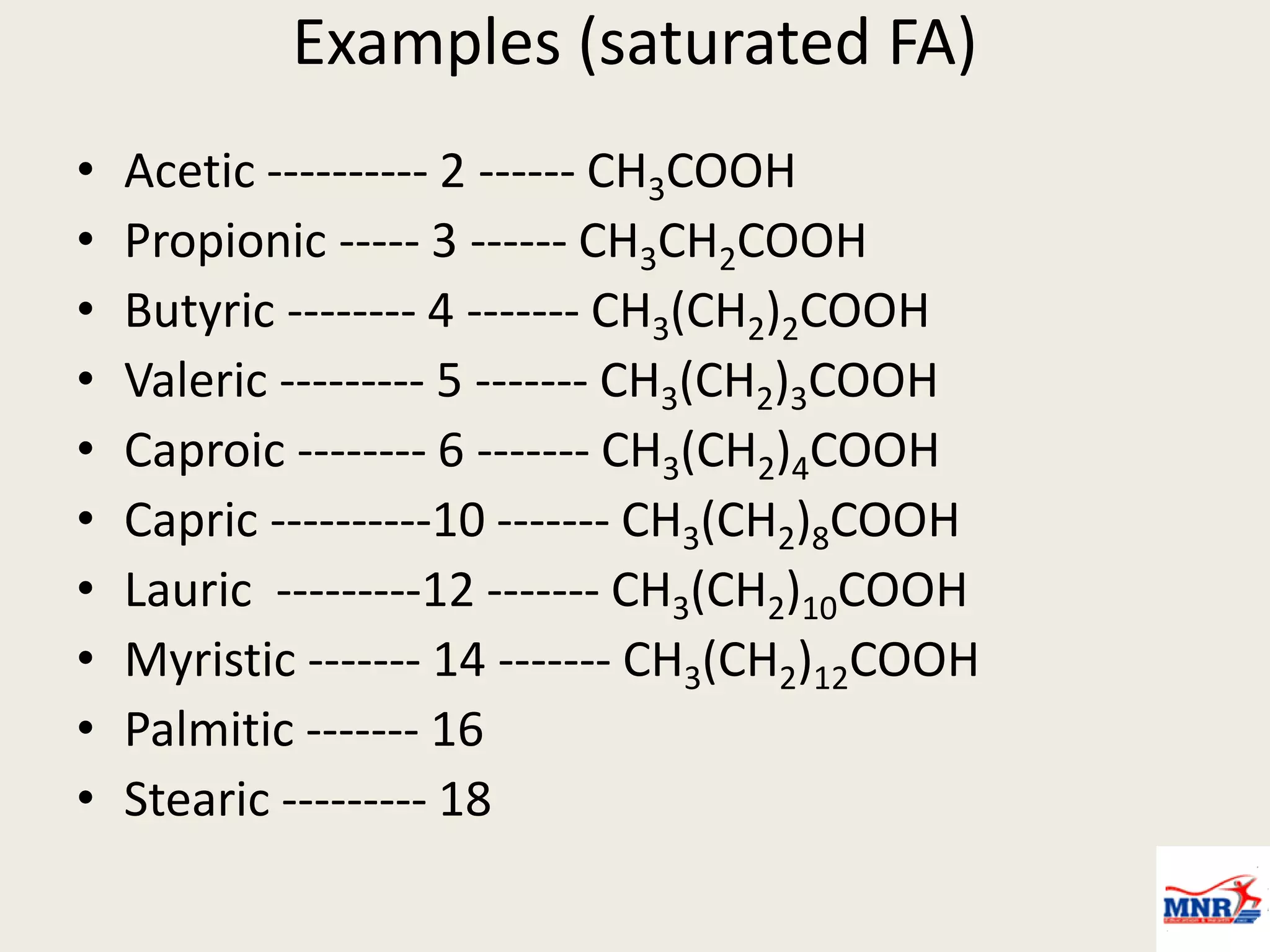
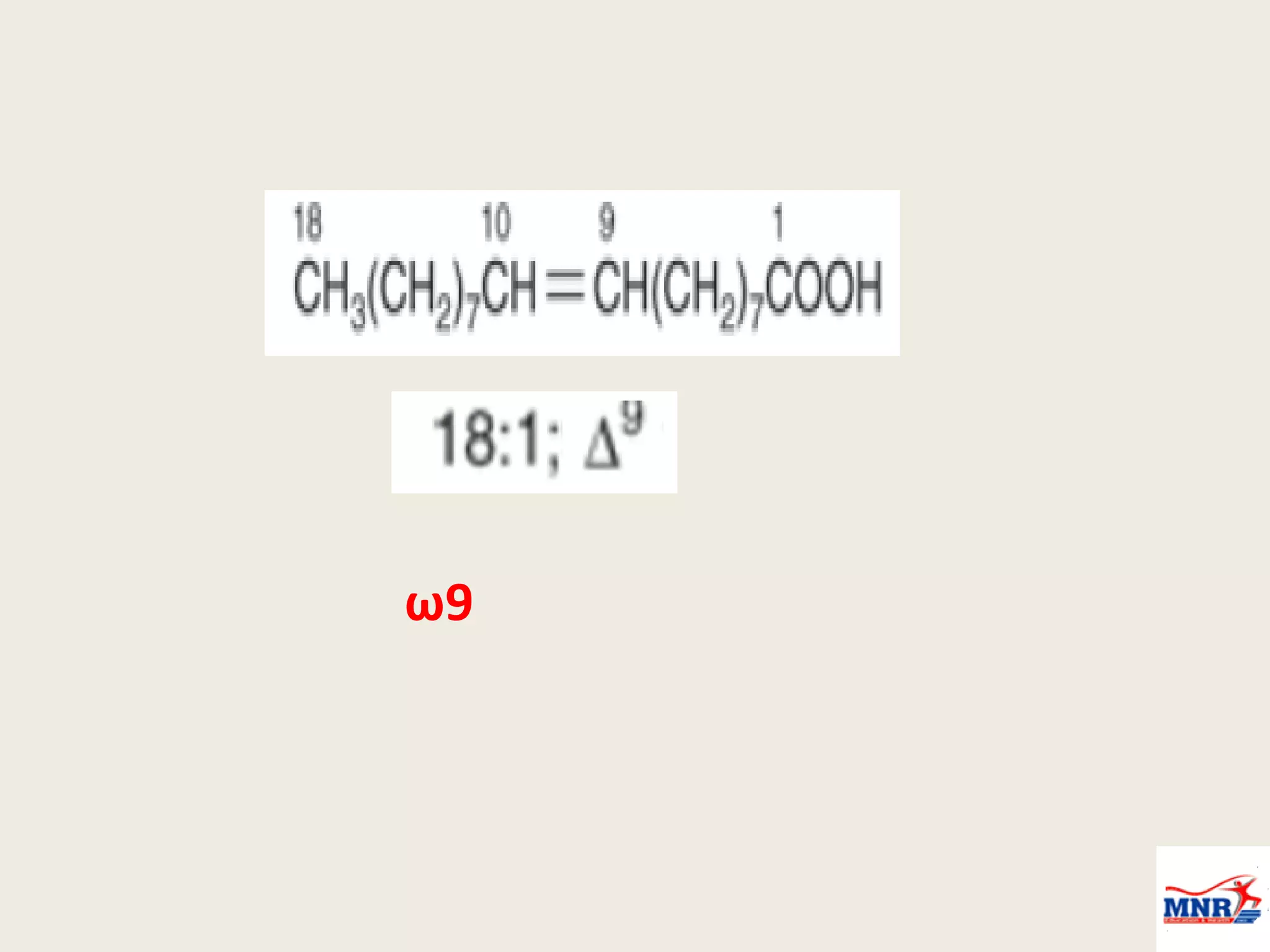
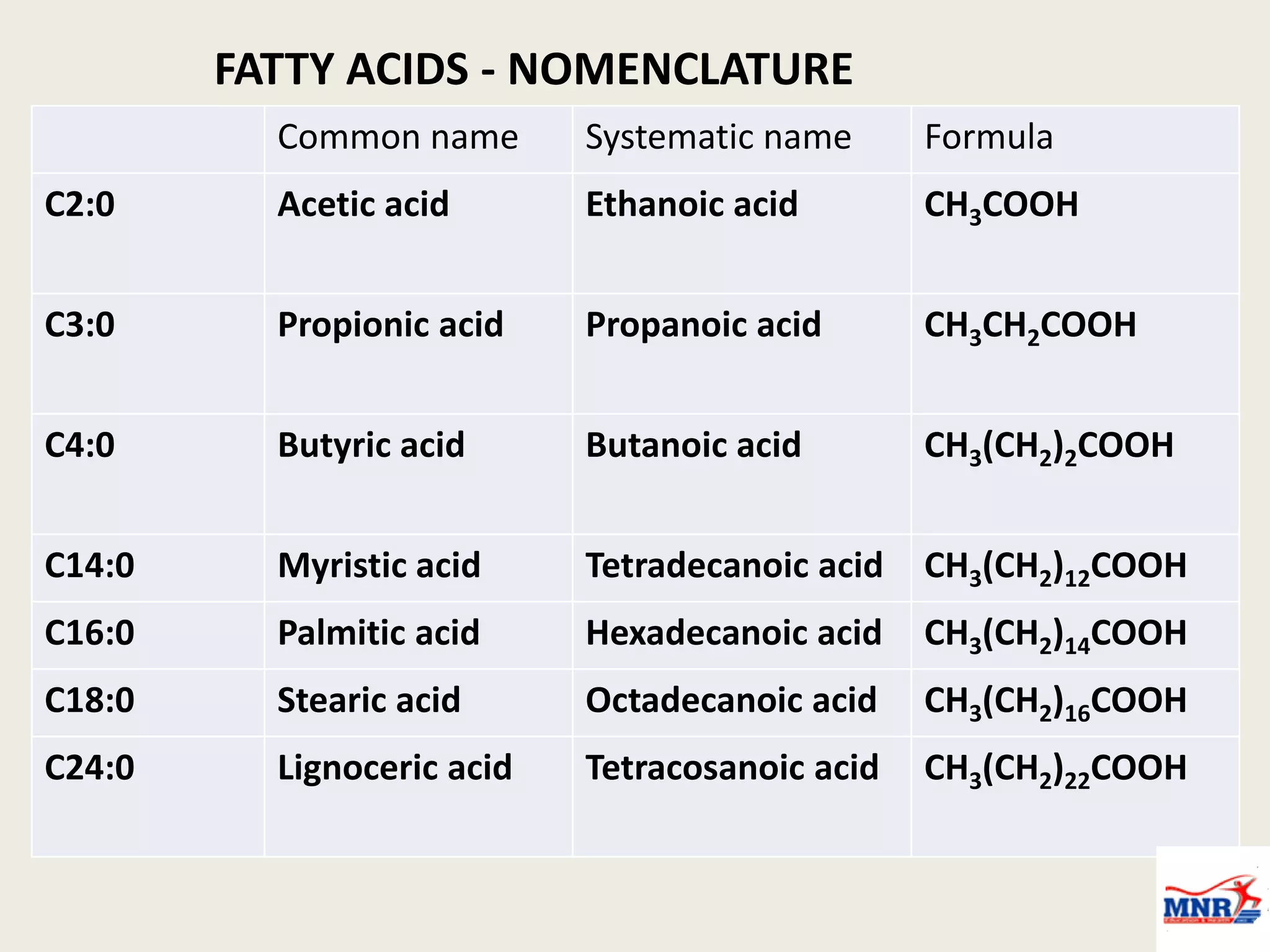
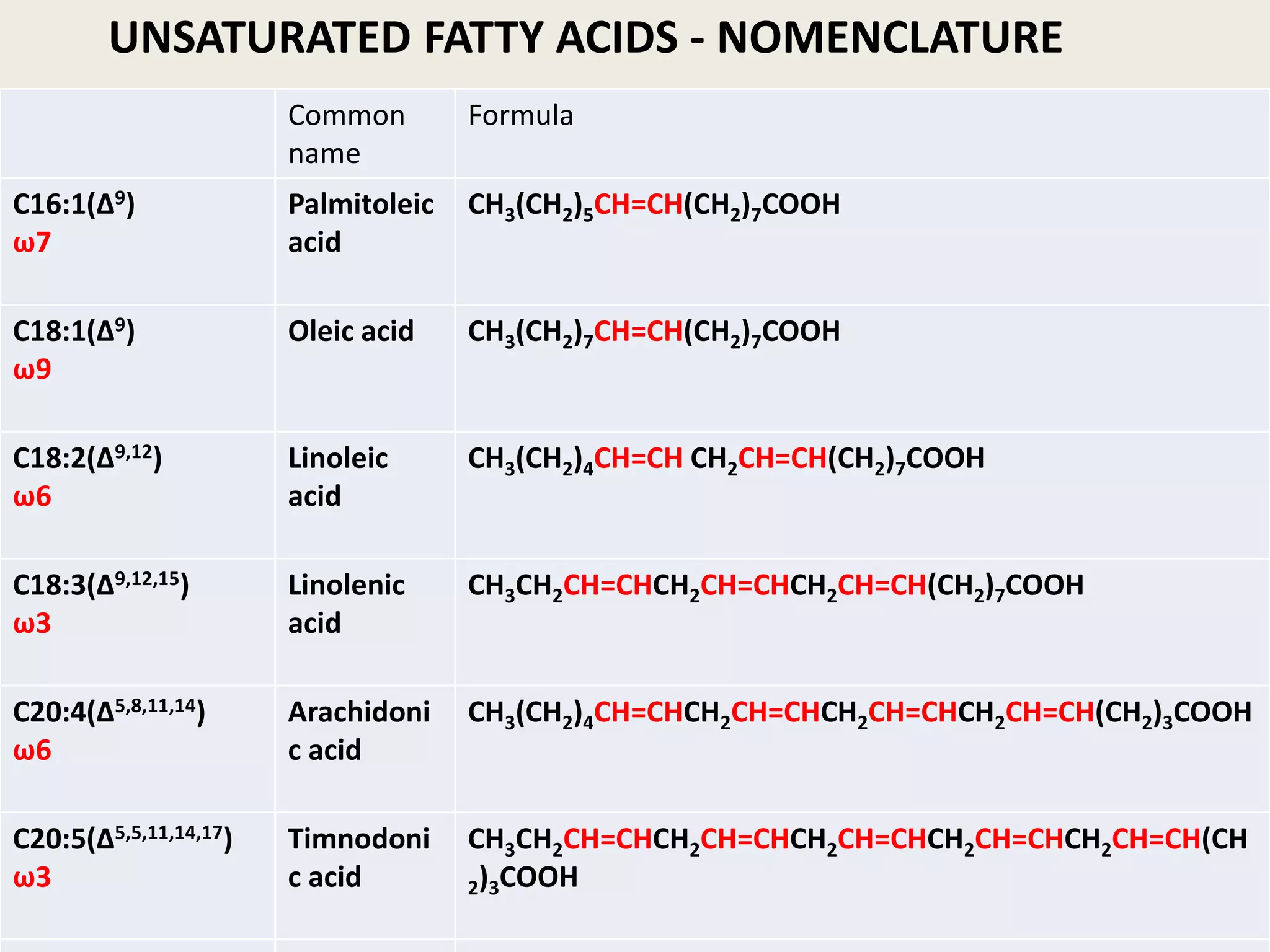
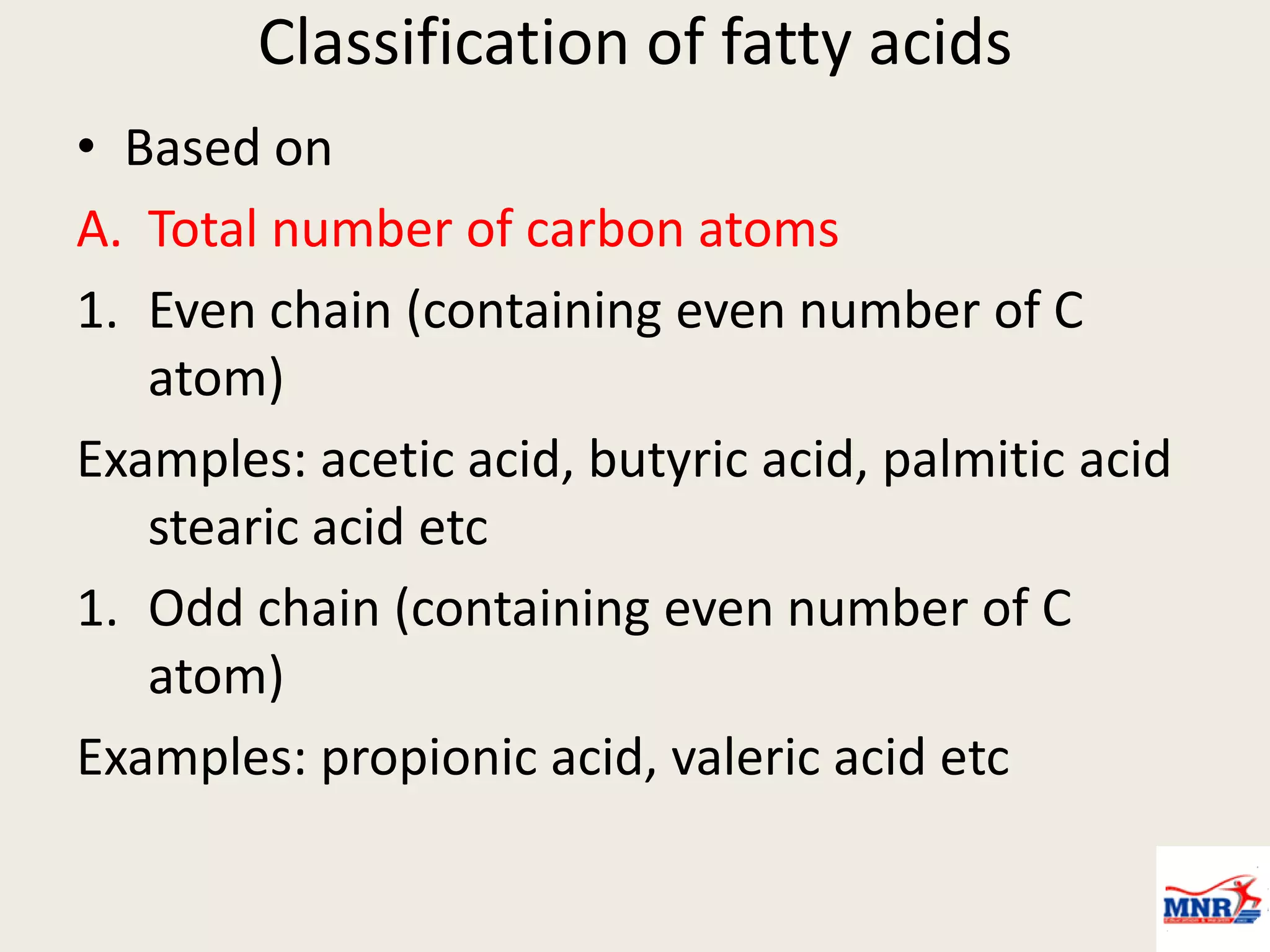
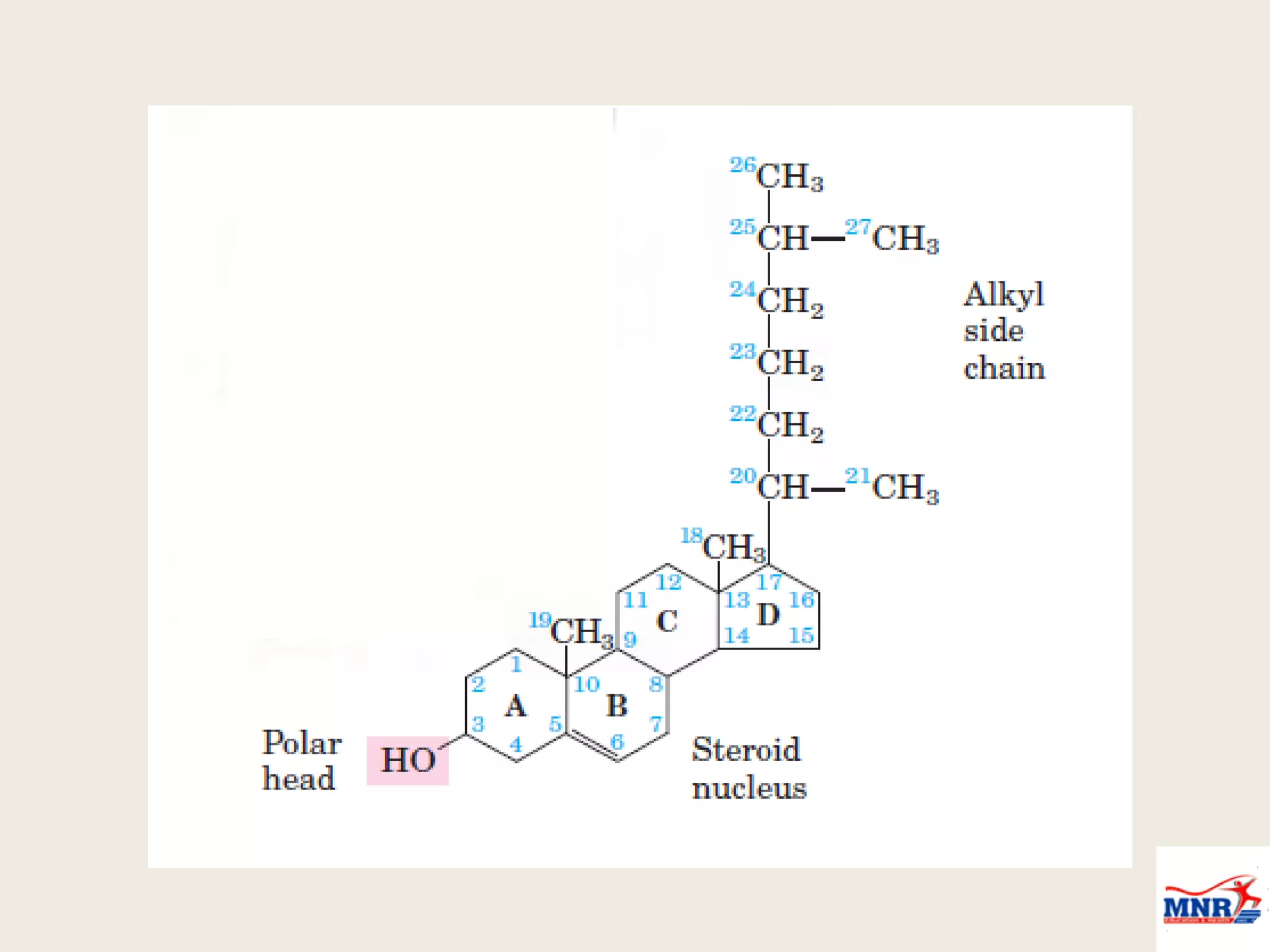
The document discusses lipid chemistry, covering the definition, functions, classification, and significance of lipids in biological systems. It explains various types of lipids, such as simple lipids, complex lipids, and derived lipids, along with their roles in health and disease. Additionally, it highlights the clinical implications of lipids in conditions like obesity, cardiovascular diseases, and diabetes mellitus.