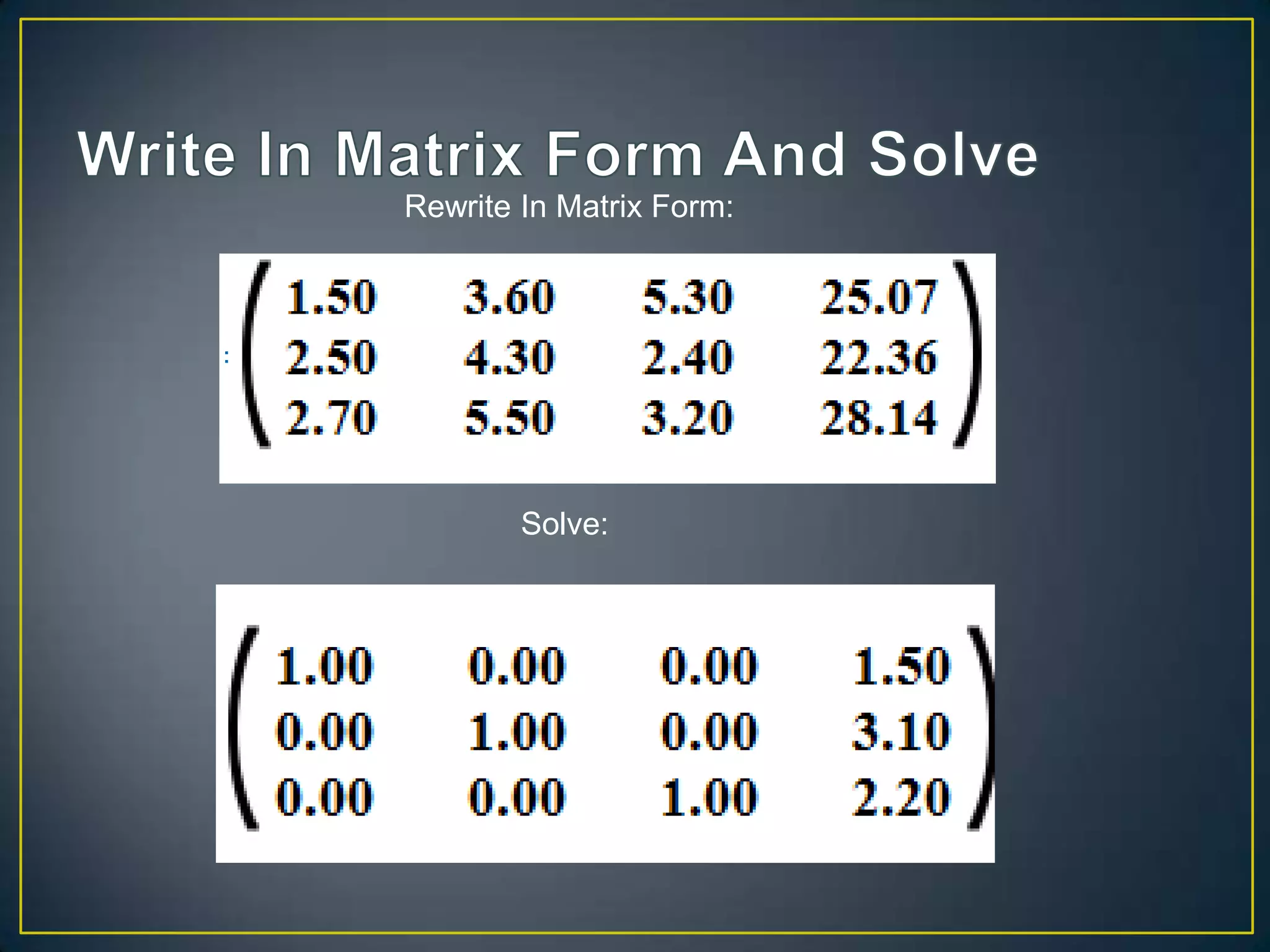
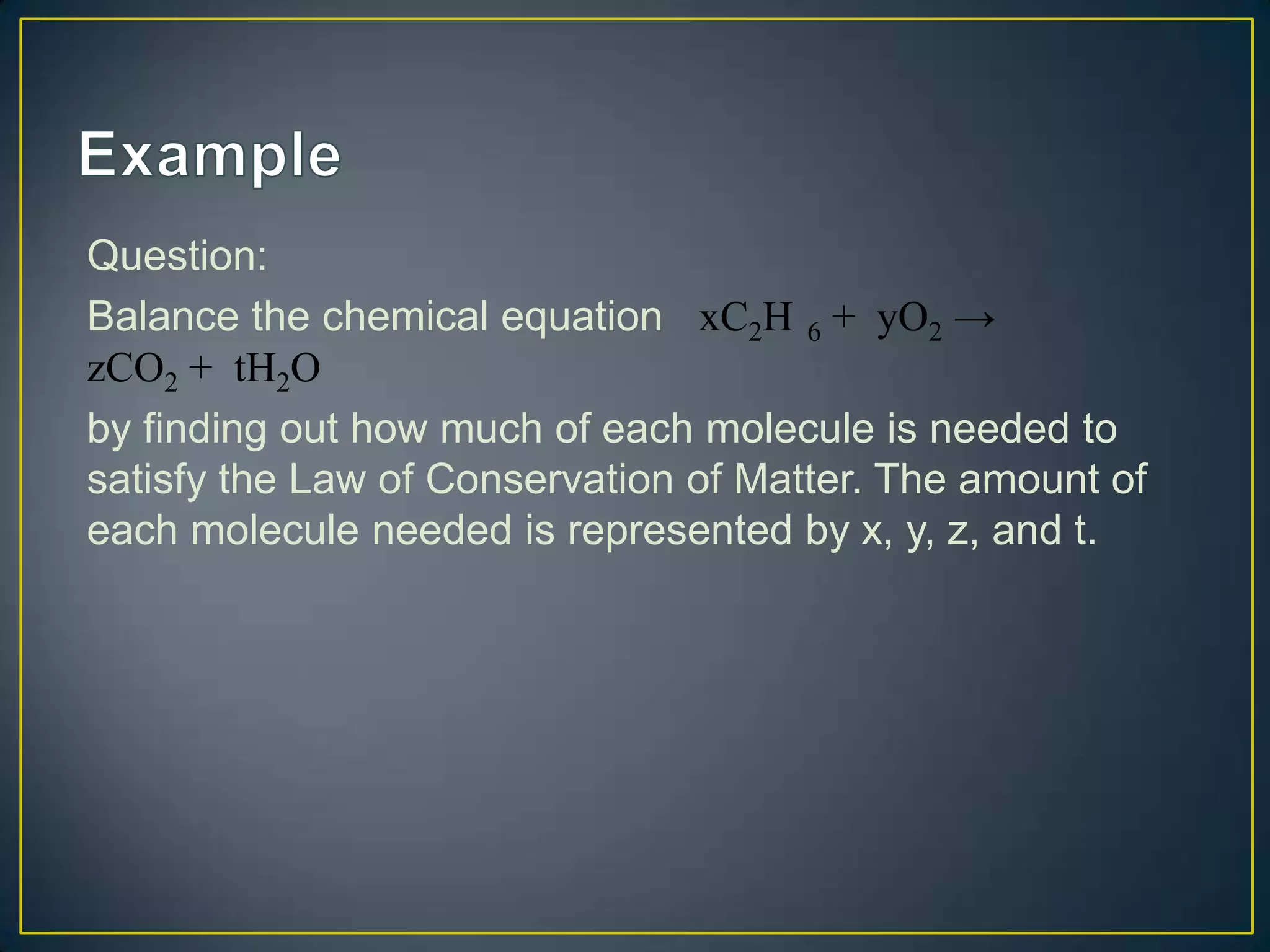
The document discusses using linear algebra to solve chemistry problems involving balancing chemical equations and determining volumes of chemical solutions. It provides an example of using a system of equations represented in matrix form to determine that the volumes of solutions A, B, and C are 1.5 cm3, 3.1 cm3, and 2.2 cm3 based on the amounts of chemical produced under different concentration conditions. The document also demonstrates how to use the law of conservation of matter and a similar technique to balance the chemical equation C2H6 + O2 → CO2 + H2O.