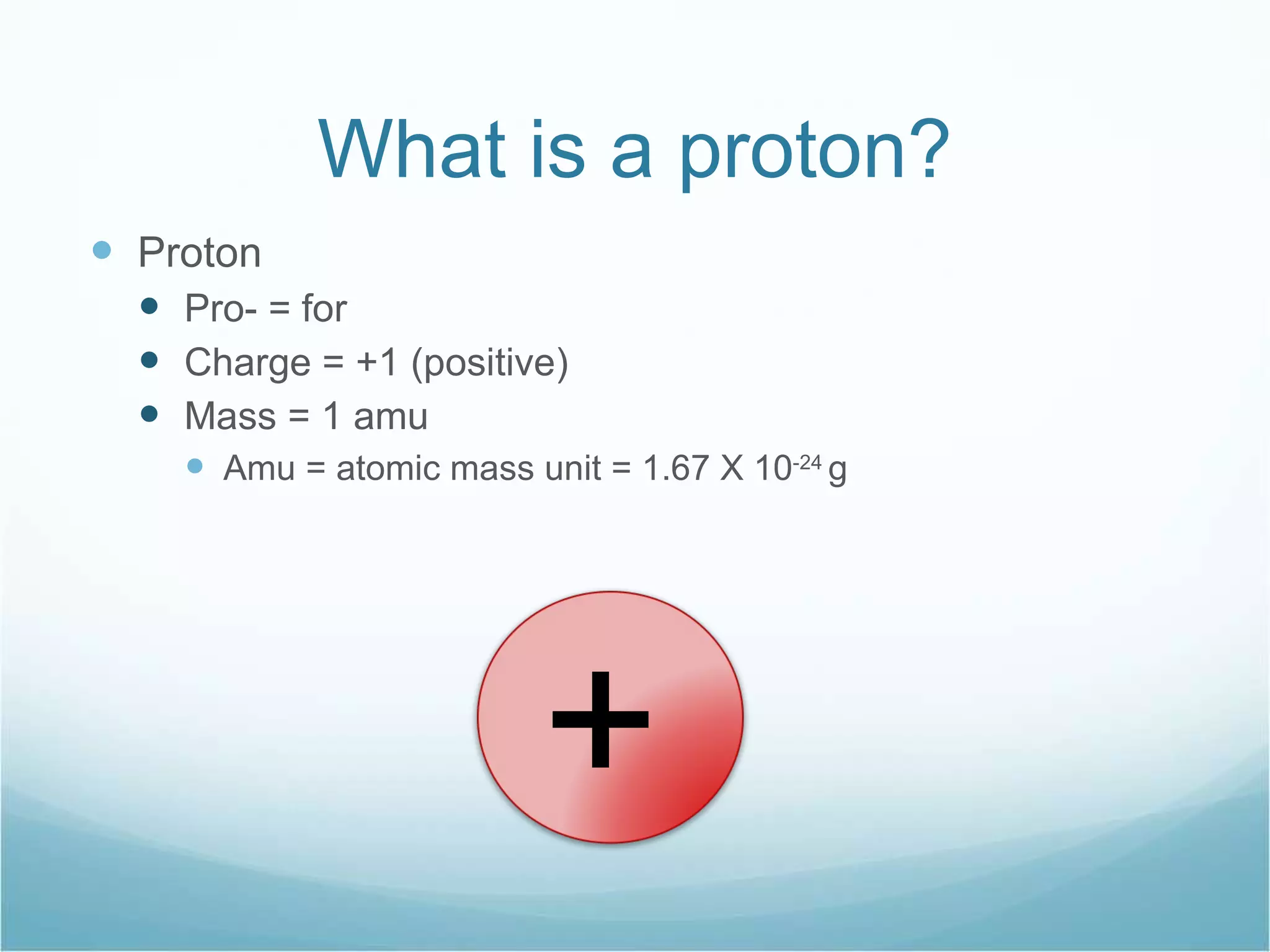
The document discusses the composition of atoms. It states that atoms are made up of three subatomic particles: protons, neutrons, and electrons. It provides details on the properties of each particle, including their charge (protons are positive, electrons are negative, neutrons have no charge) and mass (protons and neutrons have a mass of approximately 1 atomic mass unit, electrons have virtually no mass).