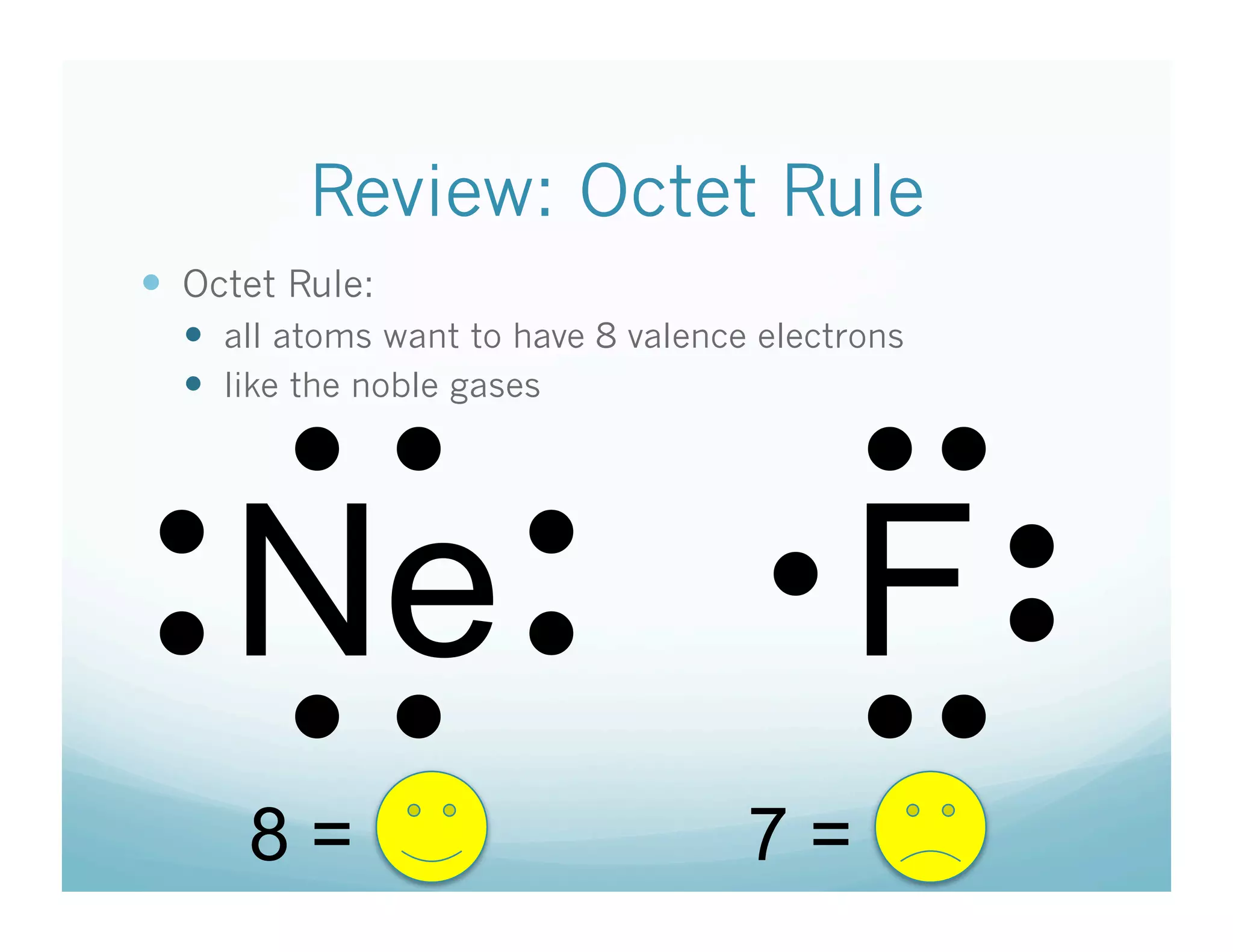
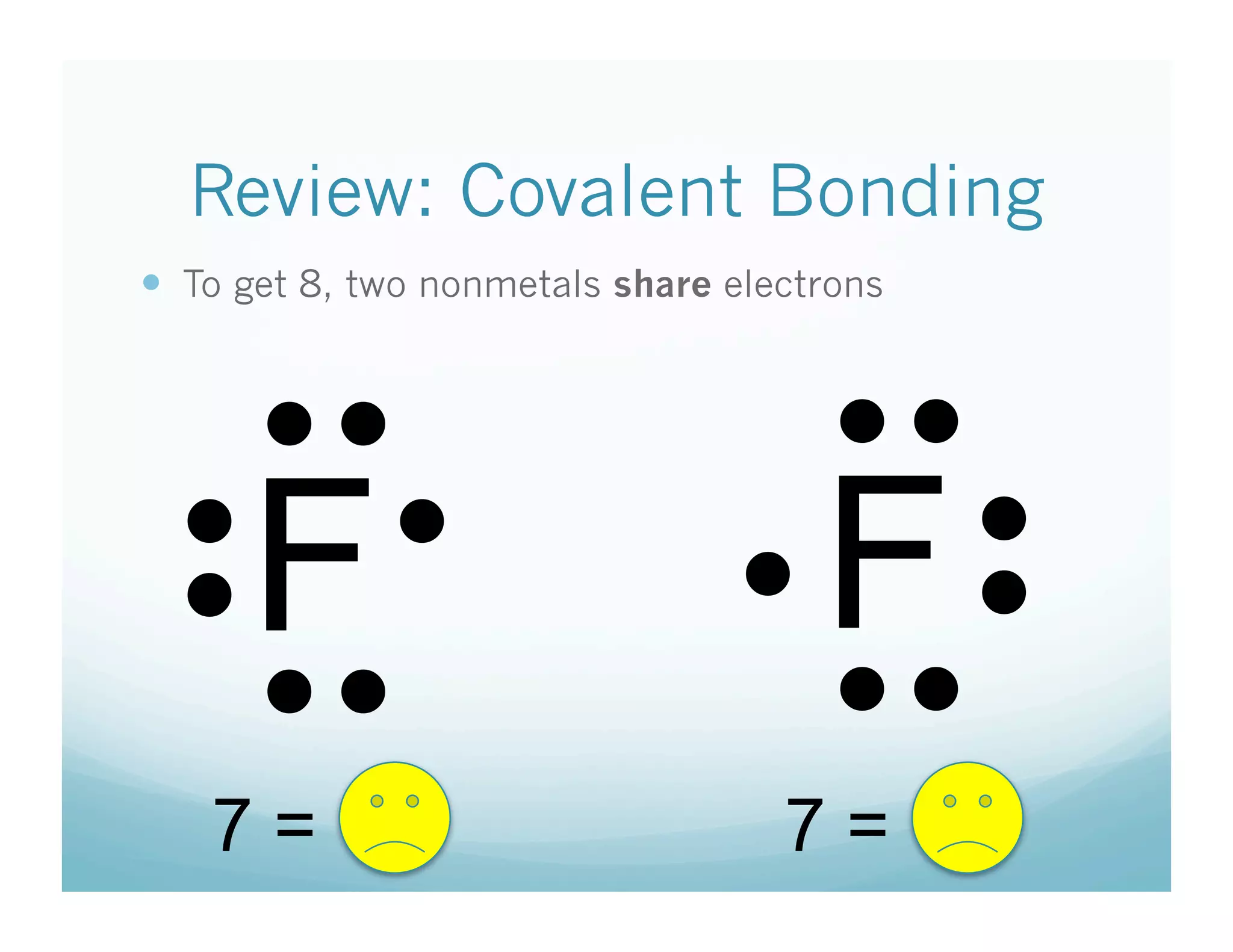
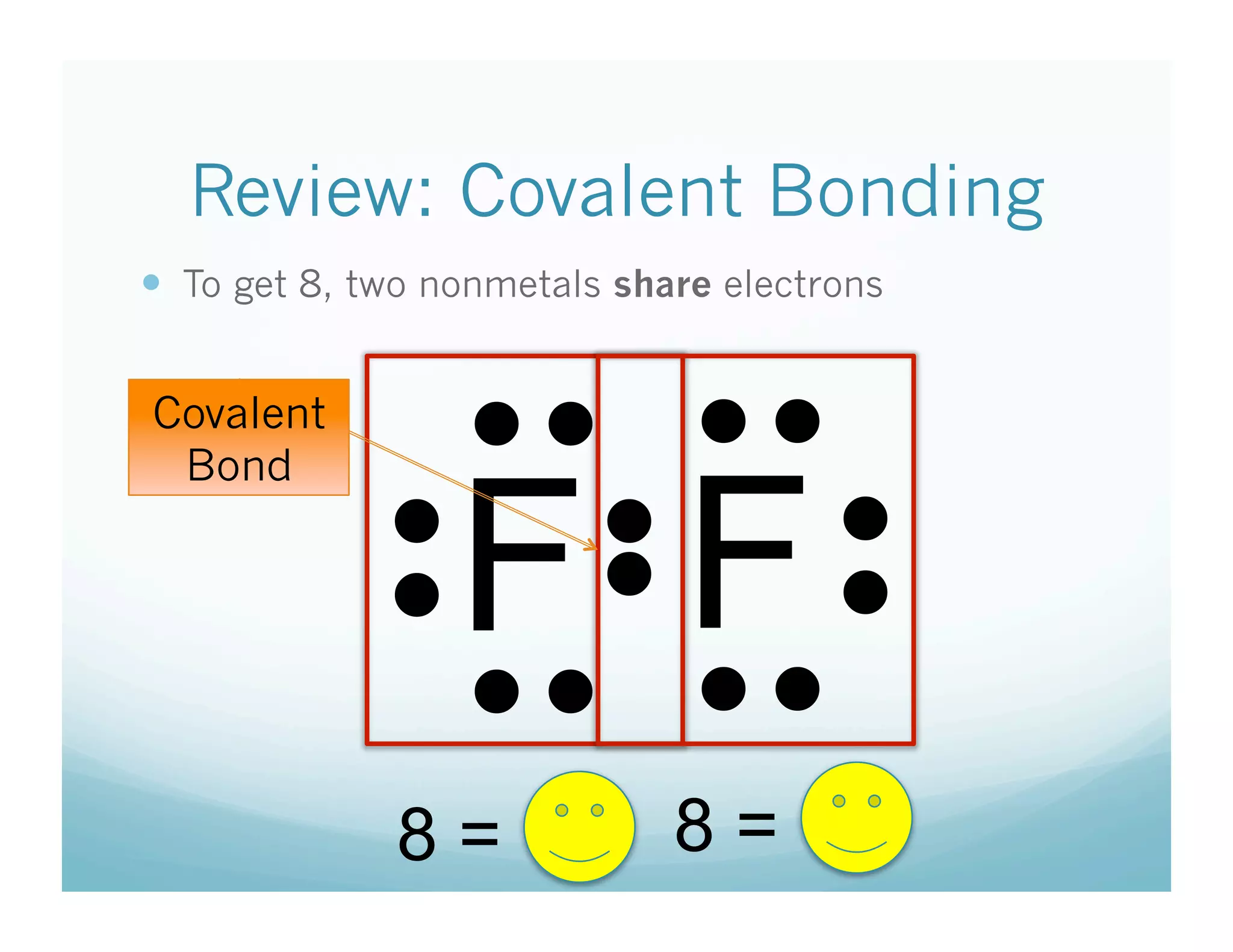
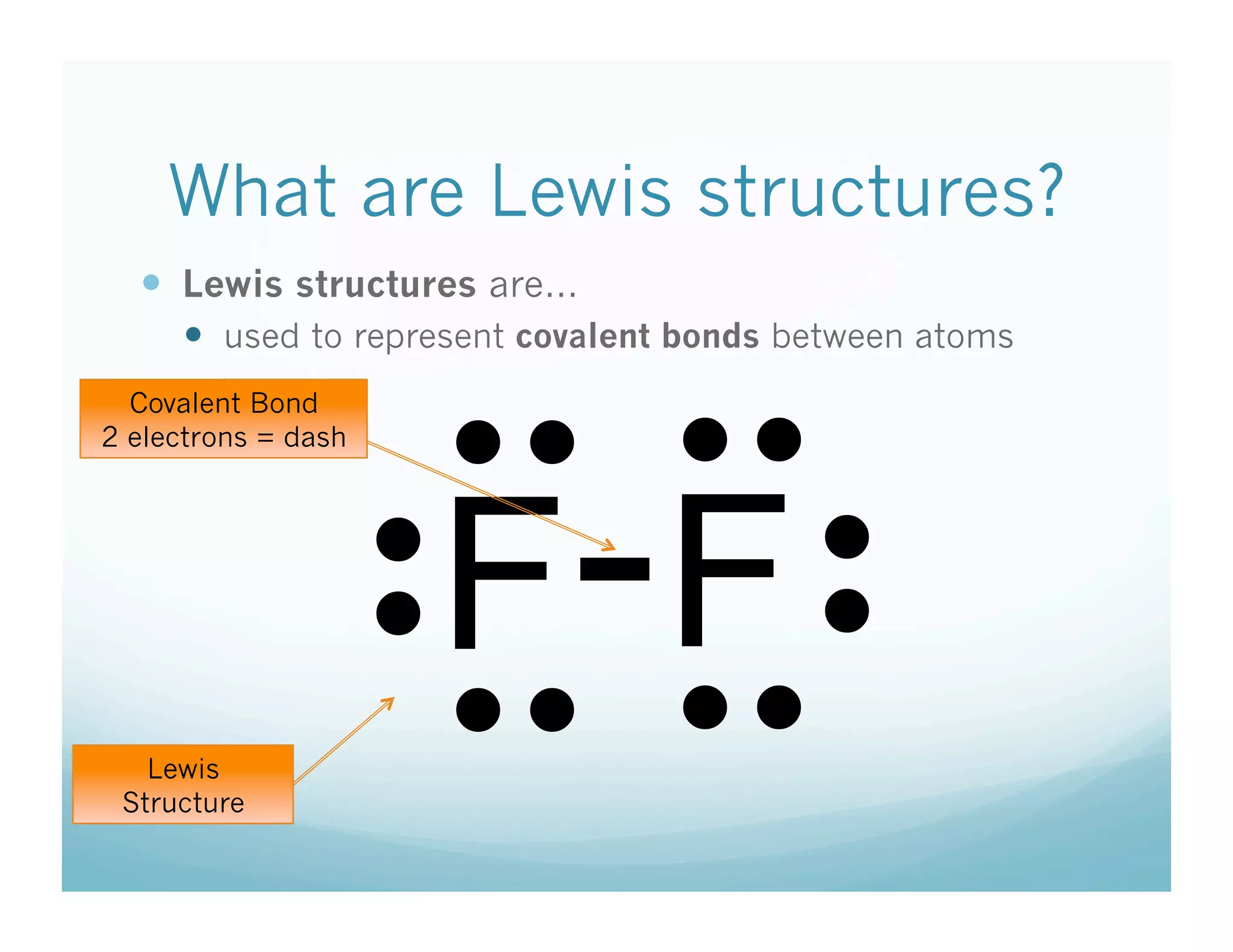
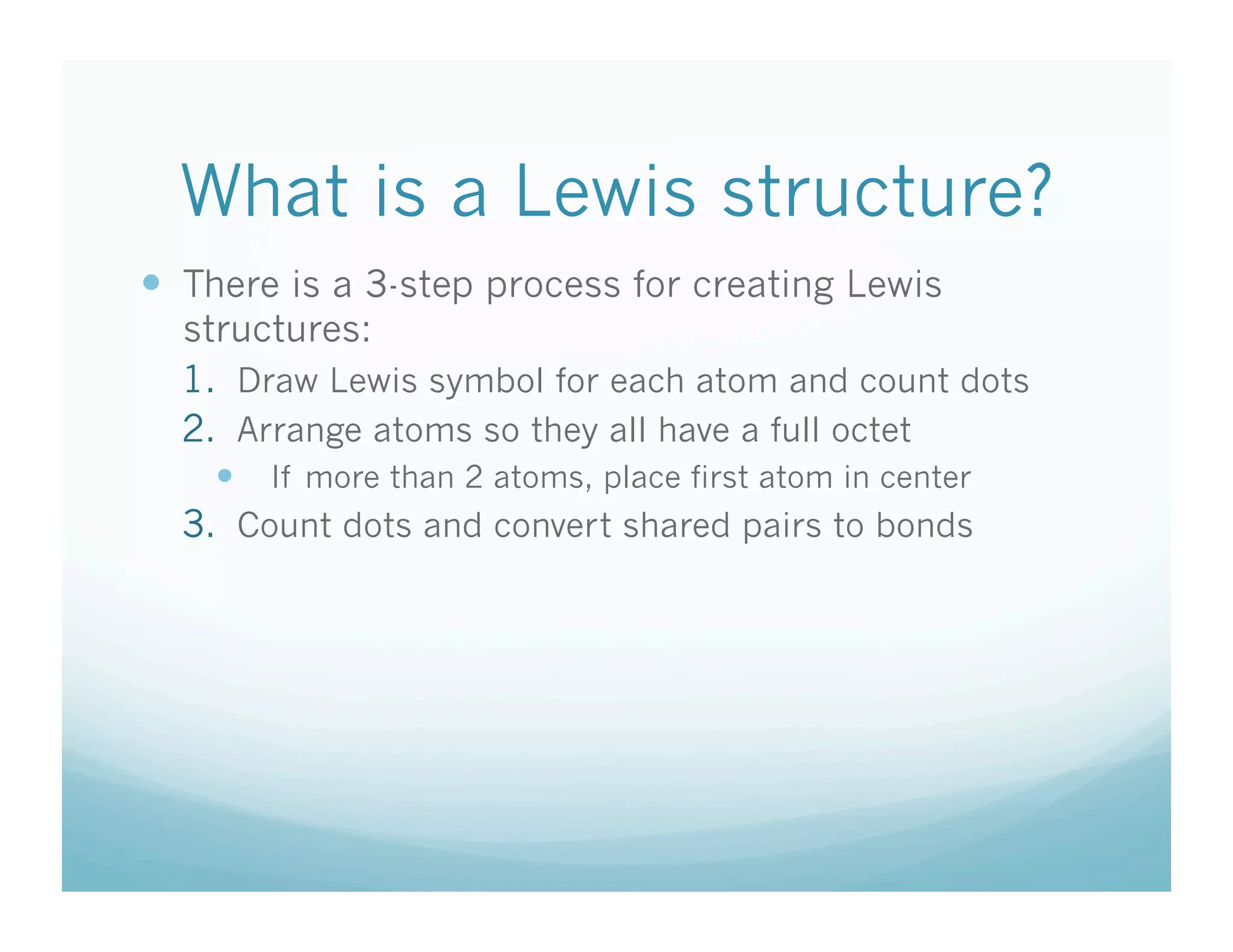
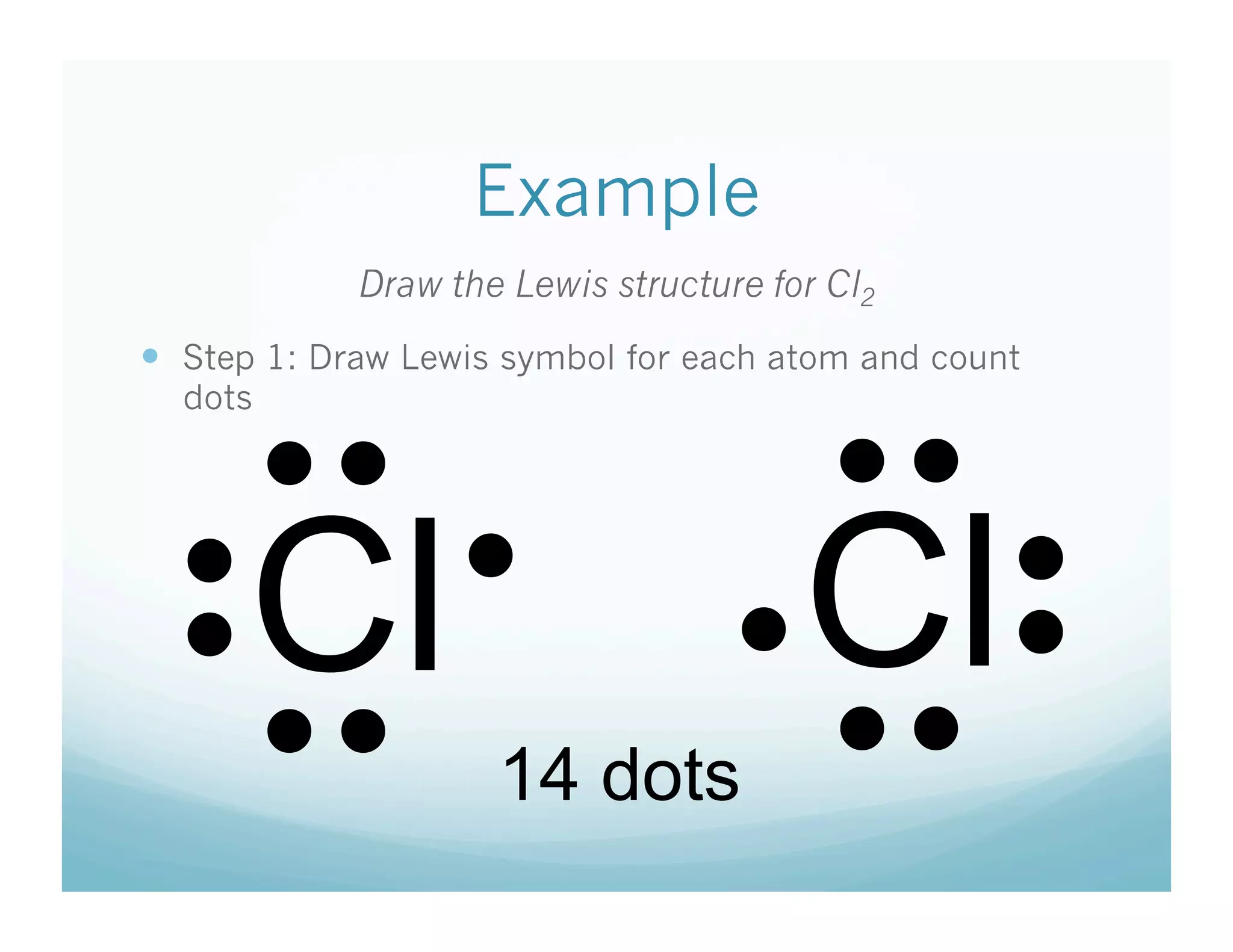
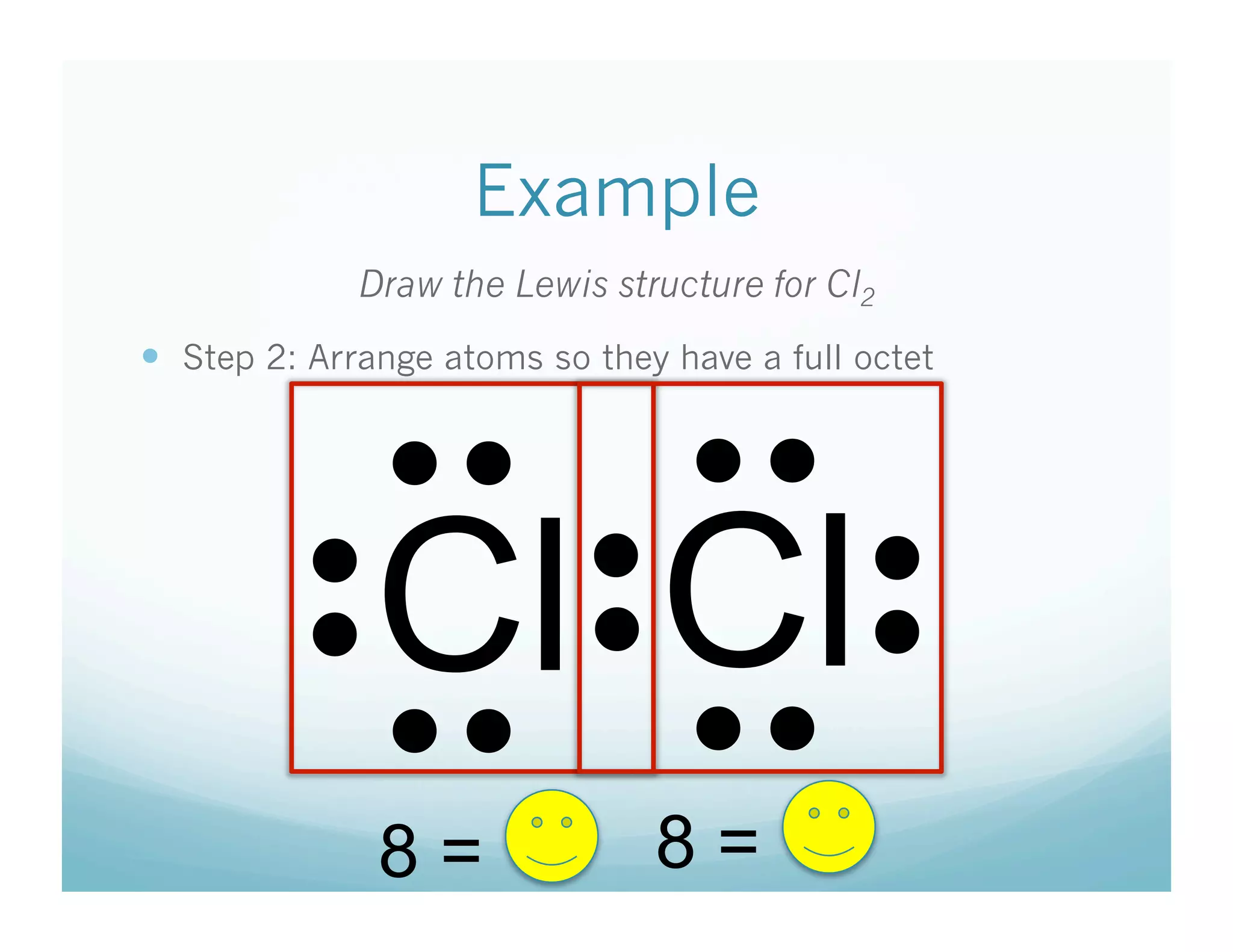
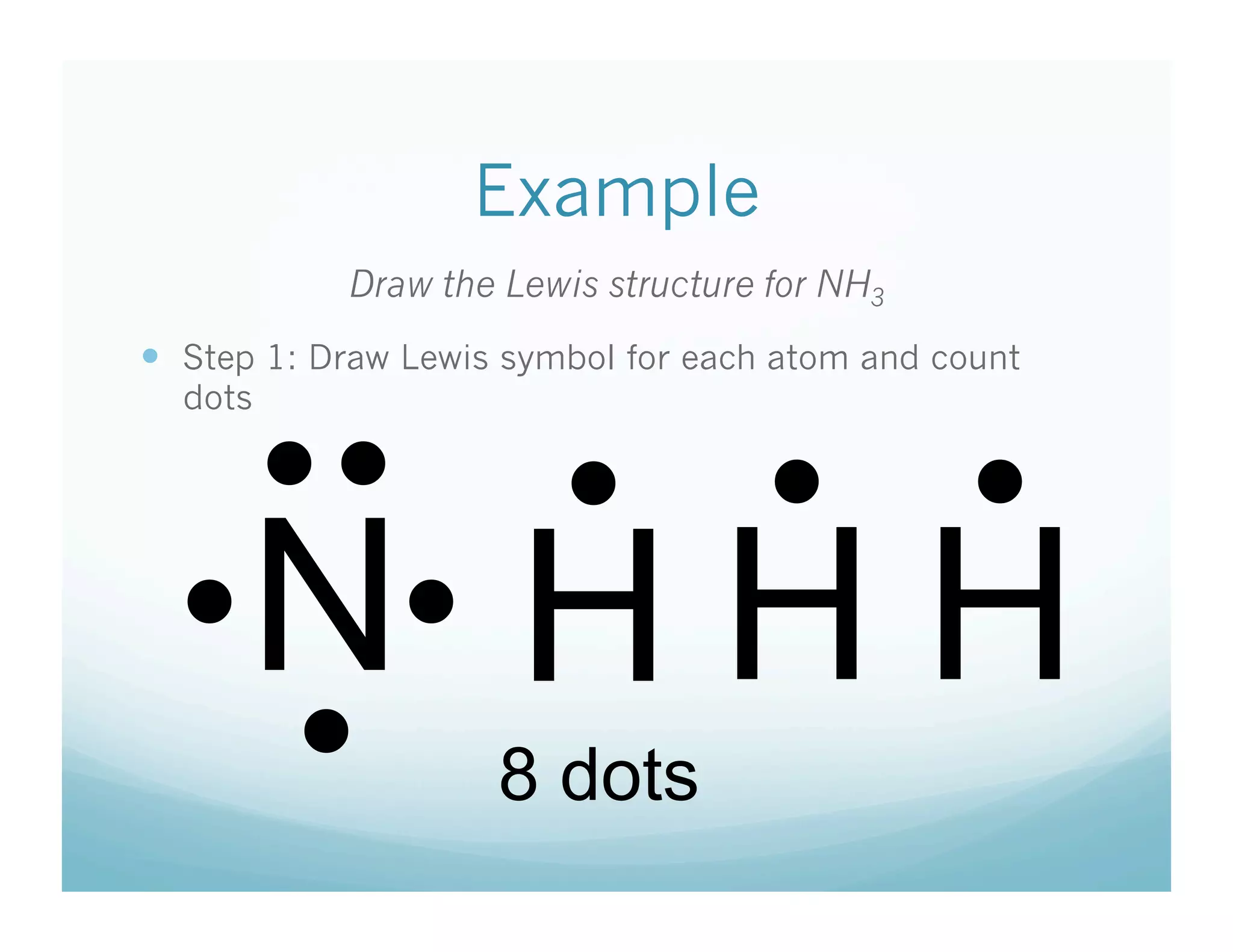
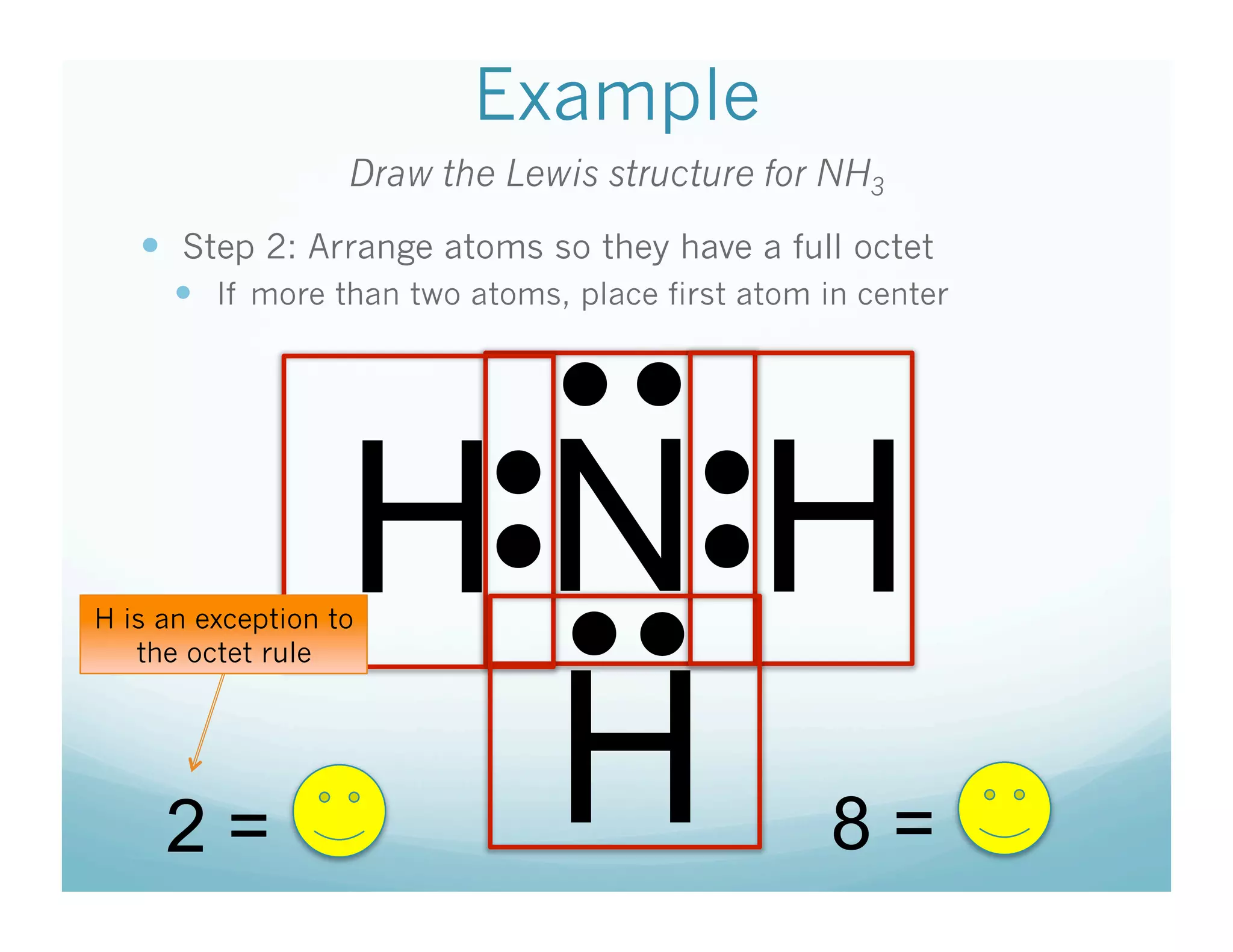
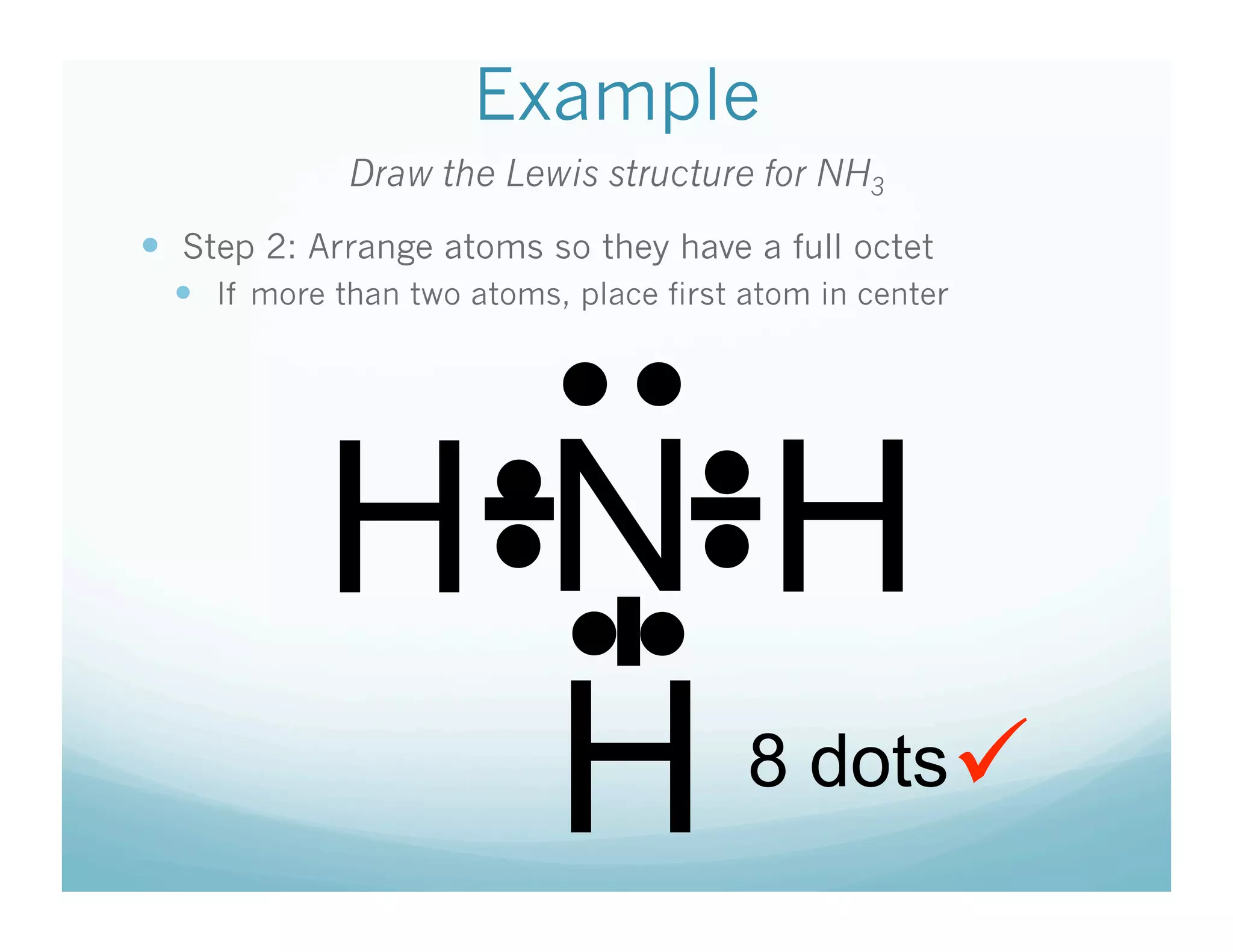
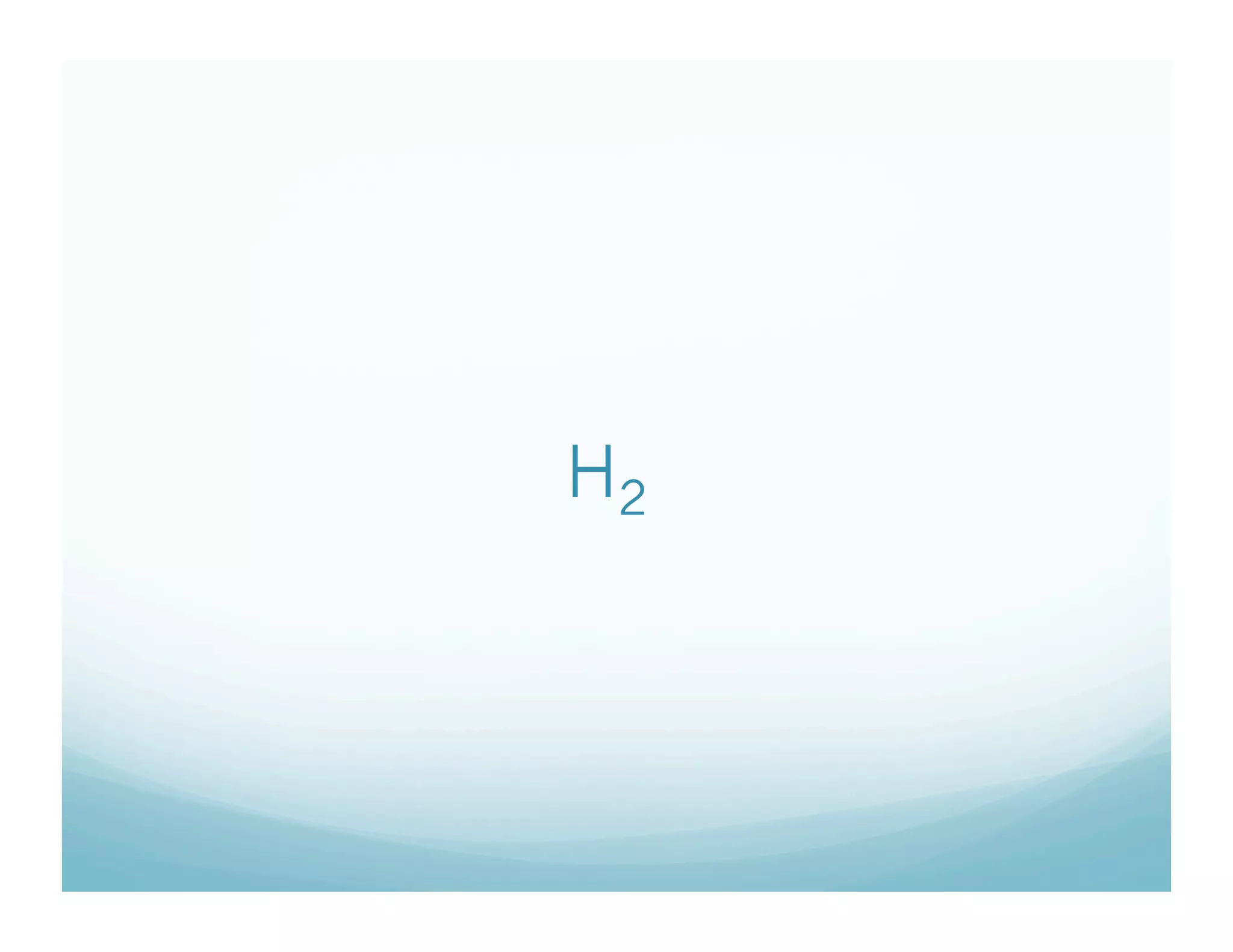
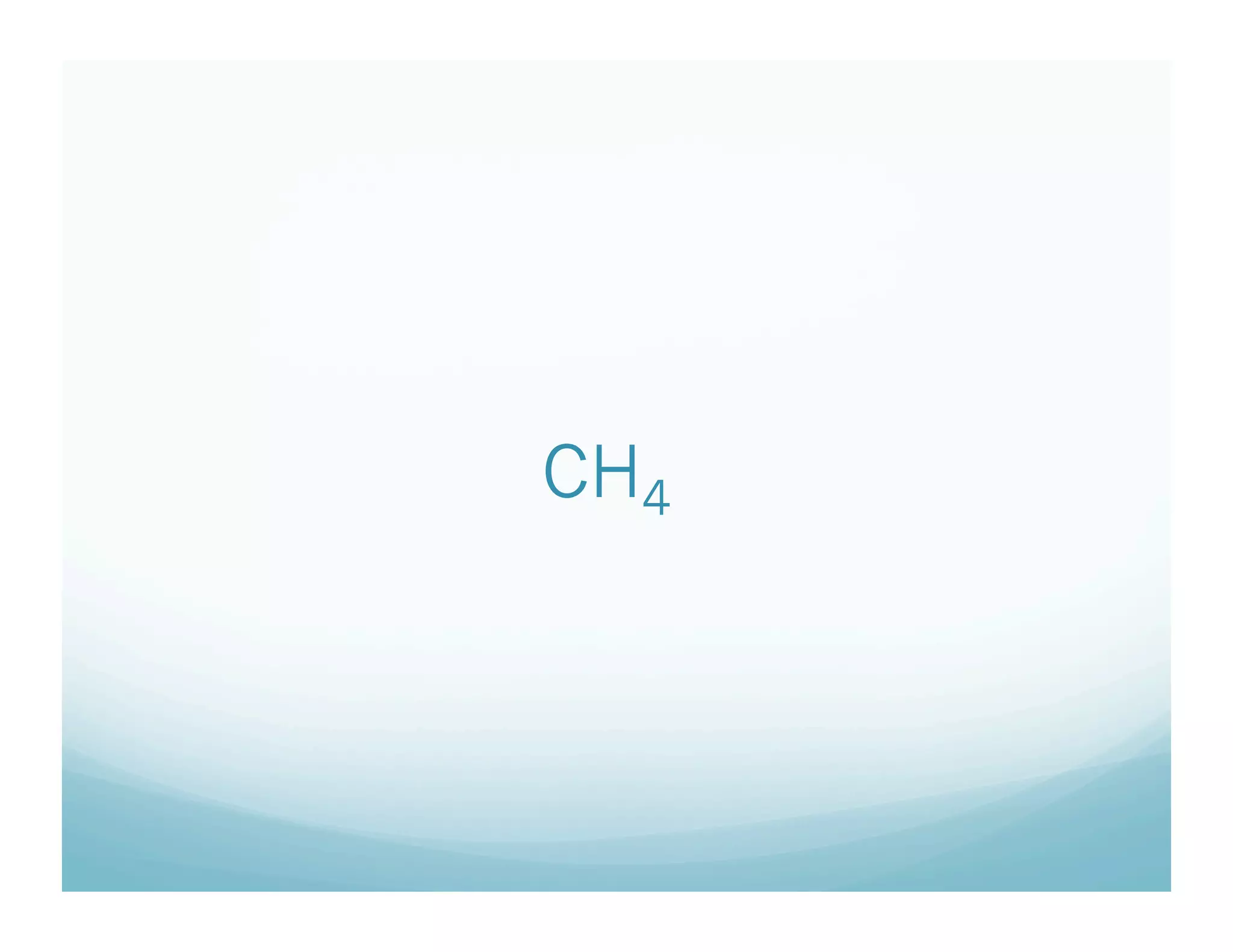
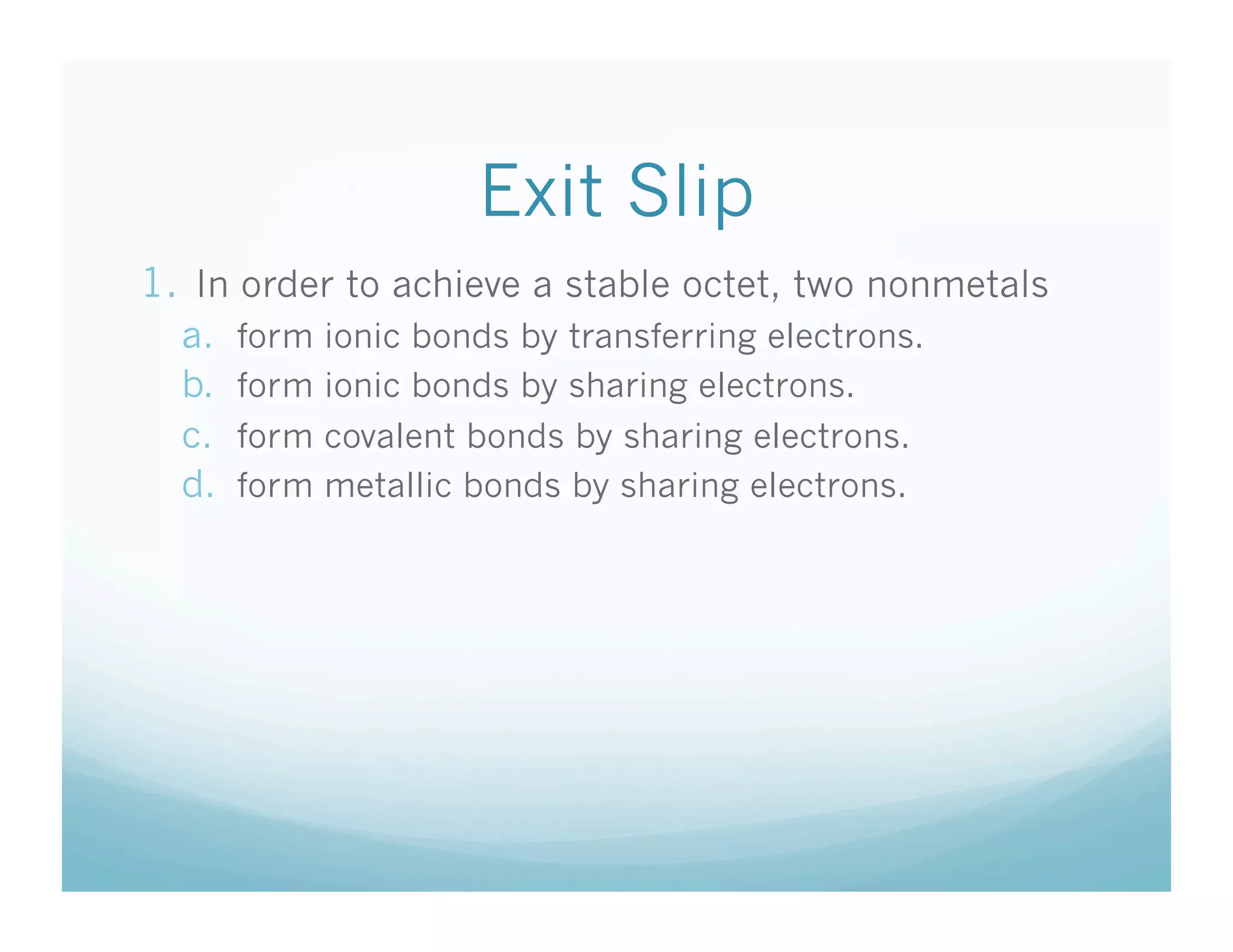
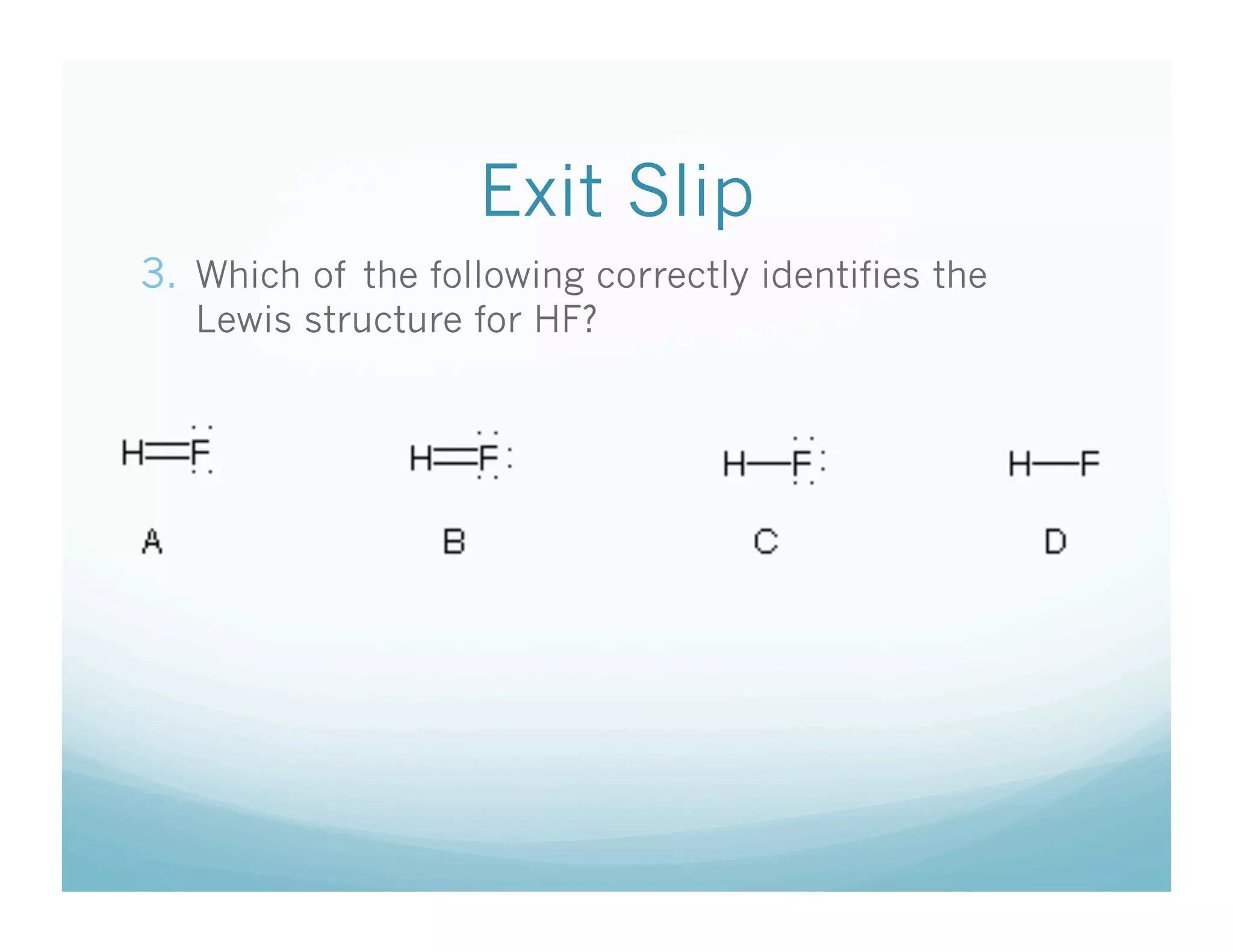
This document provides an agenda and lesson plan for a chemistry class covering covalent bonding and Lewis structures over the course of a week. The schedule lists the daily topics as covalent bonds from Monday to Thursday with a review on Friday and another review the following Monday before a unit exam on Tuesday. The document explains the three step process for drawing Lewis structures: 1) draw Lewis symbols, 2) arrange atoms to achieve a full octet, 3) convert shared electron pairs to bonds. It provides examples of drawing Lewis structures for diatomic molecules like Cl2 and polyatomic molecules like NH3.