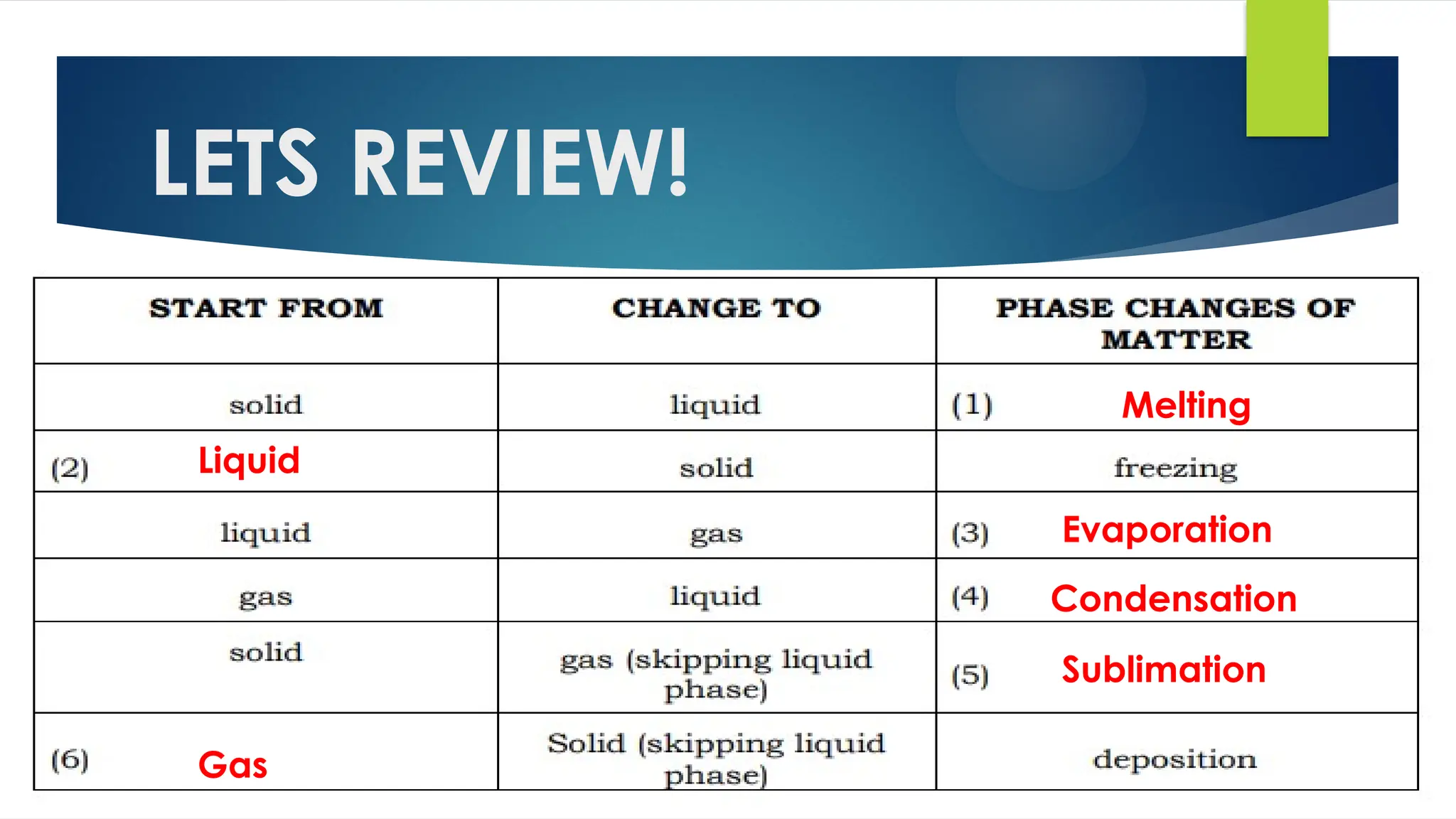
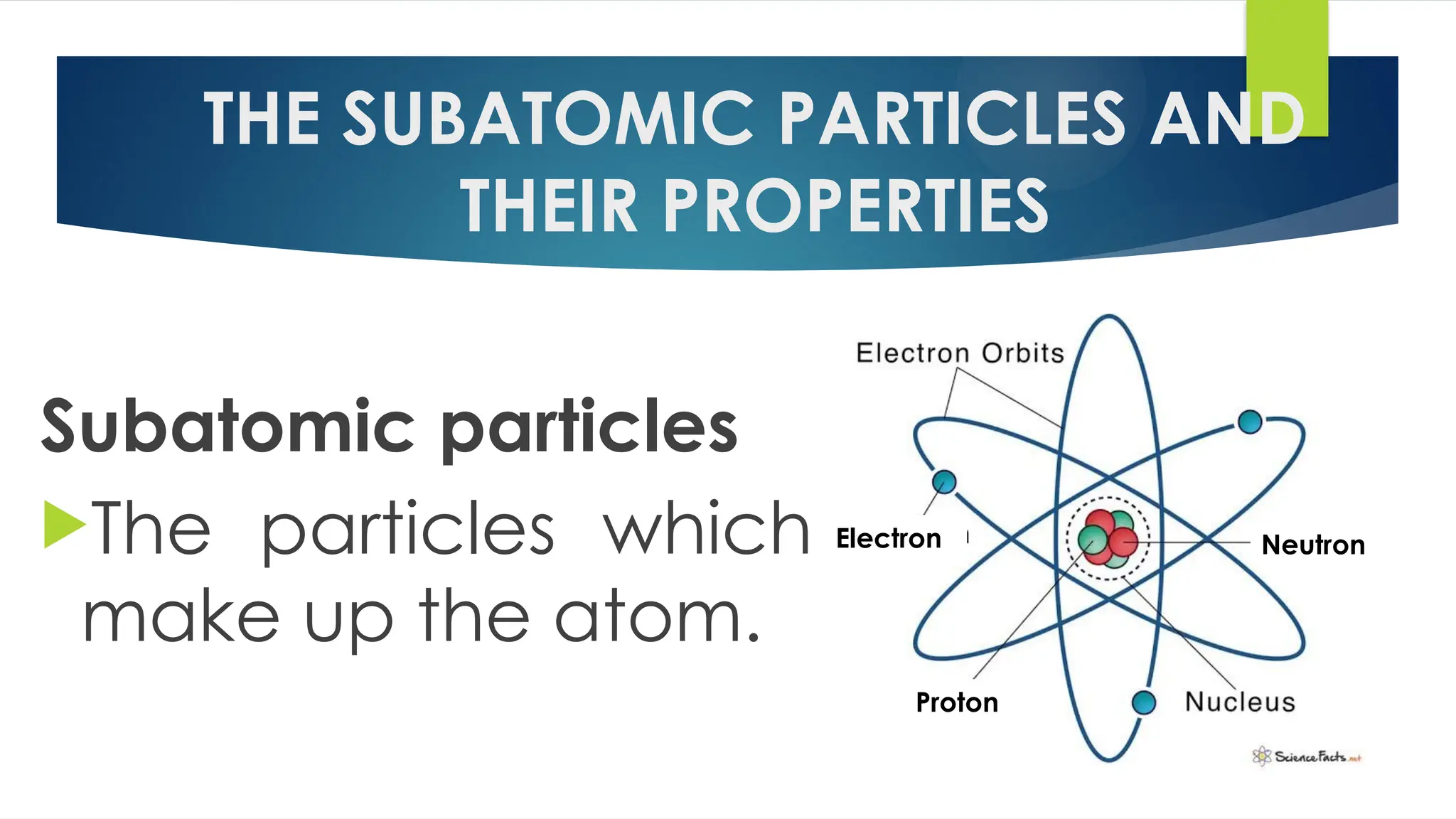
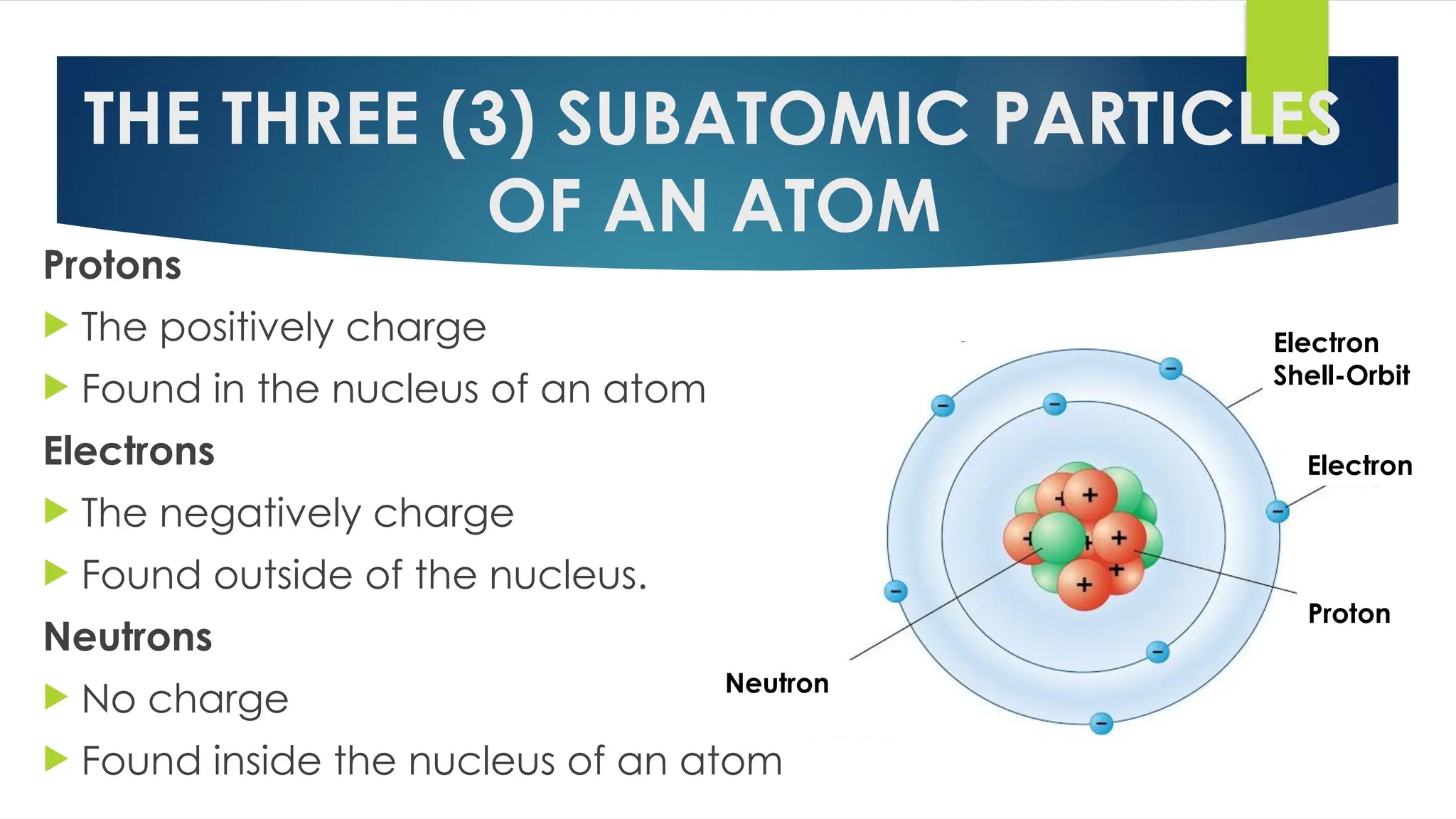
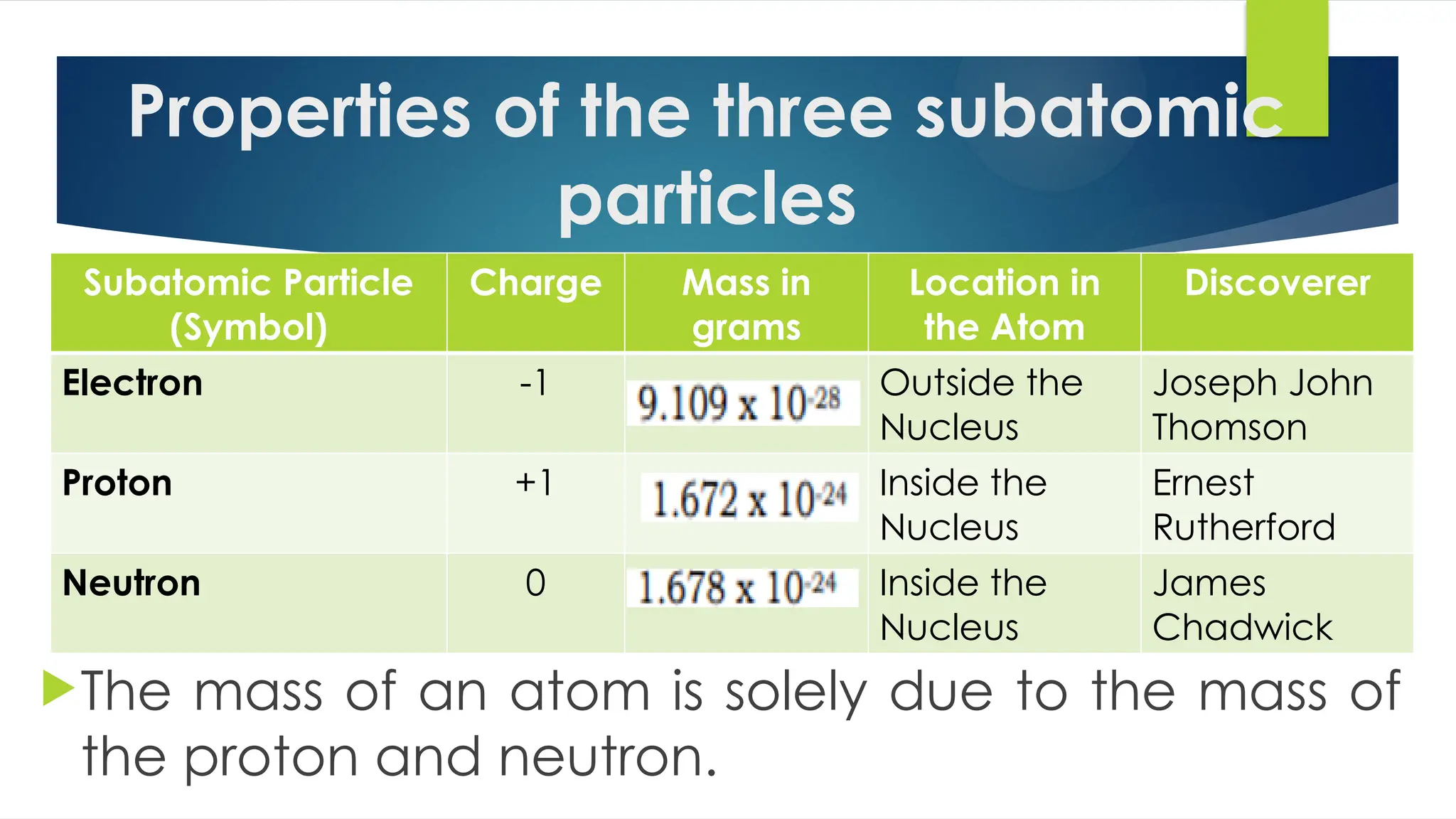
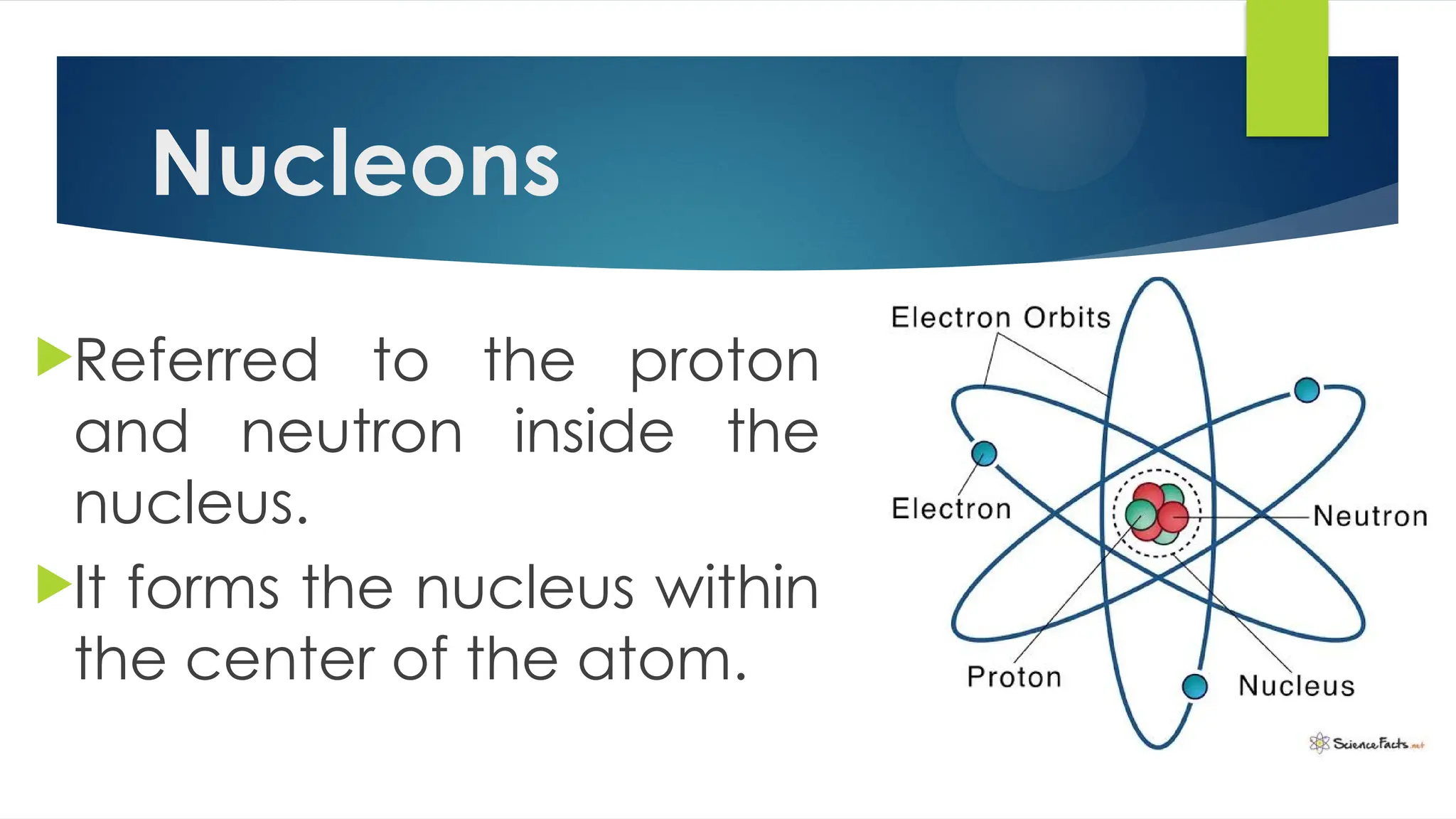
The document provides an overview of atoms and their subatomic particles, specifically electrons, protons, and neutrons, detailing their charges, masses, and locations within the atom. It also outlines classroom reminders for students and includes a review of concepts related to phase changes and materials that conduct or insulate electricity. Additionally, it suggests questions and activities for students to engage with the material.