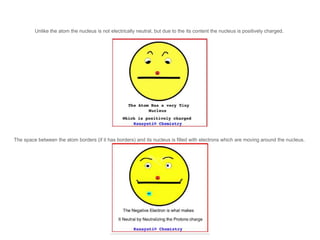
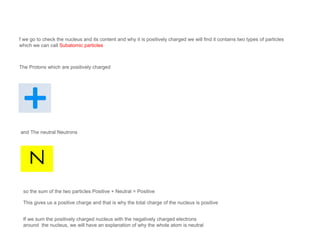
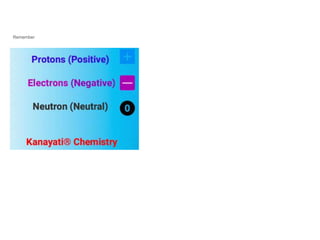
The document discusses the structure and composition of atoms, stating that atoms are the fundamental building units of matter and cannot be divided. It explains that atoms consist of a positively charged nucleus, made up of protons and neutrons, and surrounding electrons that are negatively charged. The overall charge of an atom is neutral due to the balance between the positive charge of the nucleus and the negative charge of the electrons.