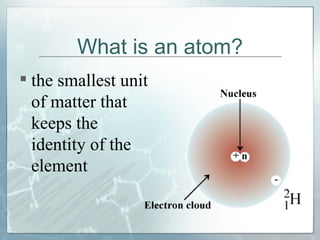
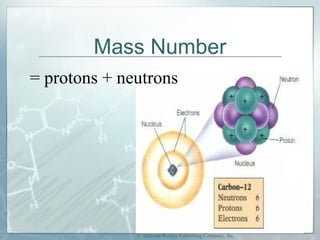
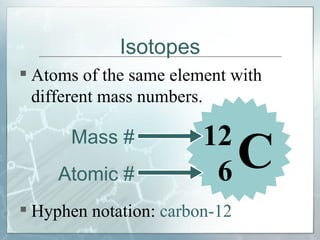
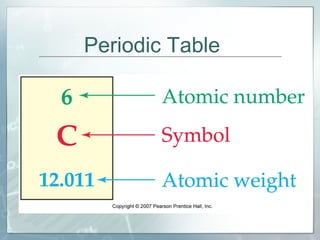
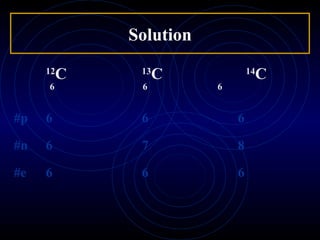
This document defines and describes subatomic particles like protons, neutrons, and electrons. It explains that atoms are made up of these subatomic particles, with protons and neutrons located in the atomic nucleus and electrons in orbitals outside the nucleus. The document also discusses atomic number, mass number, and isotopes, providing examples of writing out the number of protons, neutrons, and electrons for different atoms and ions.