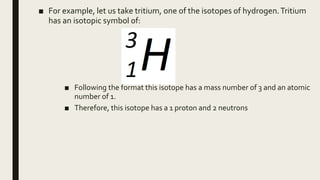
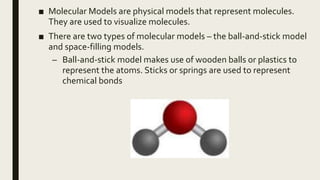
The document discusses atomic number, mass number, and isotopes. It defines atomic number as the number of protons in an atom's nucleus, which determines an element's chemical properties. Mass number is the sum of protons and neutrons. Isotopes are atoms with the same number of protons but different neutrons. Isotopes are identified by their mass number written as chemical symbol followed by mass number. The document also discusses molecular formula, empirical formula, and structural formula as examples of chemical formulas that show the number and arrangement of atoms in a compound. It defines molecular, empirical, and structural formulas and provides an example of a structural formula. Finally, it describes ball-and-stick and space-filling molecular models used to