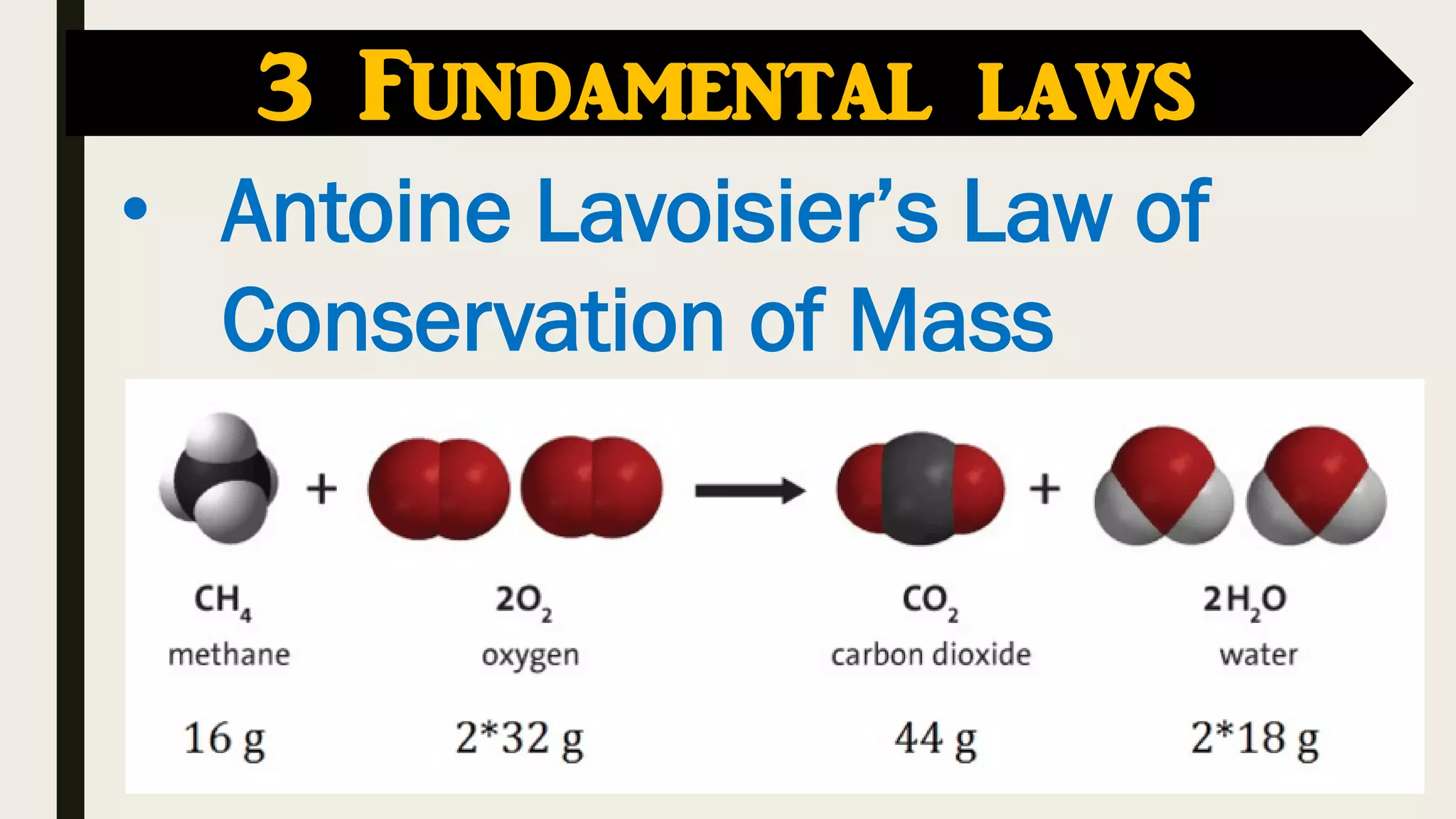
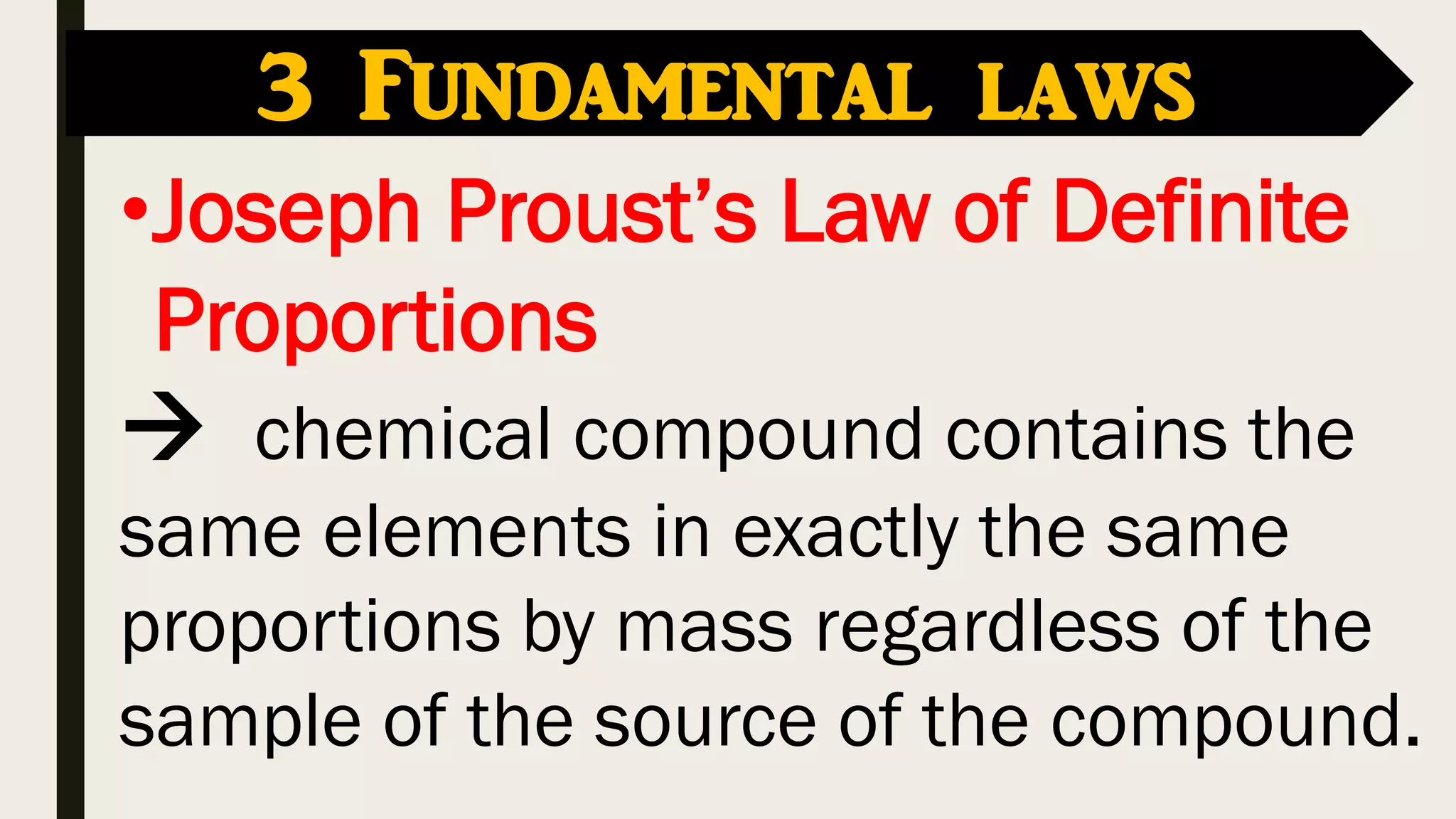
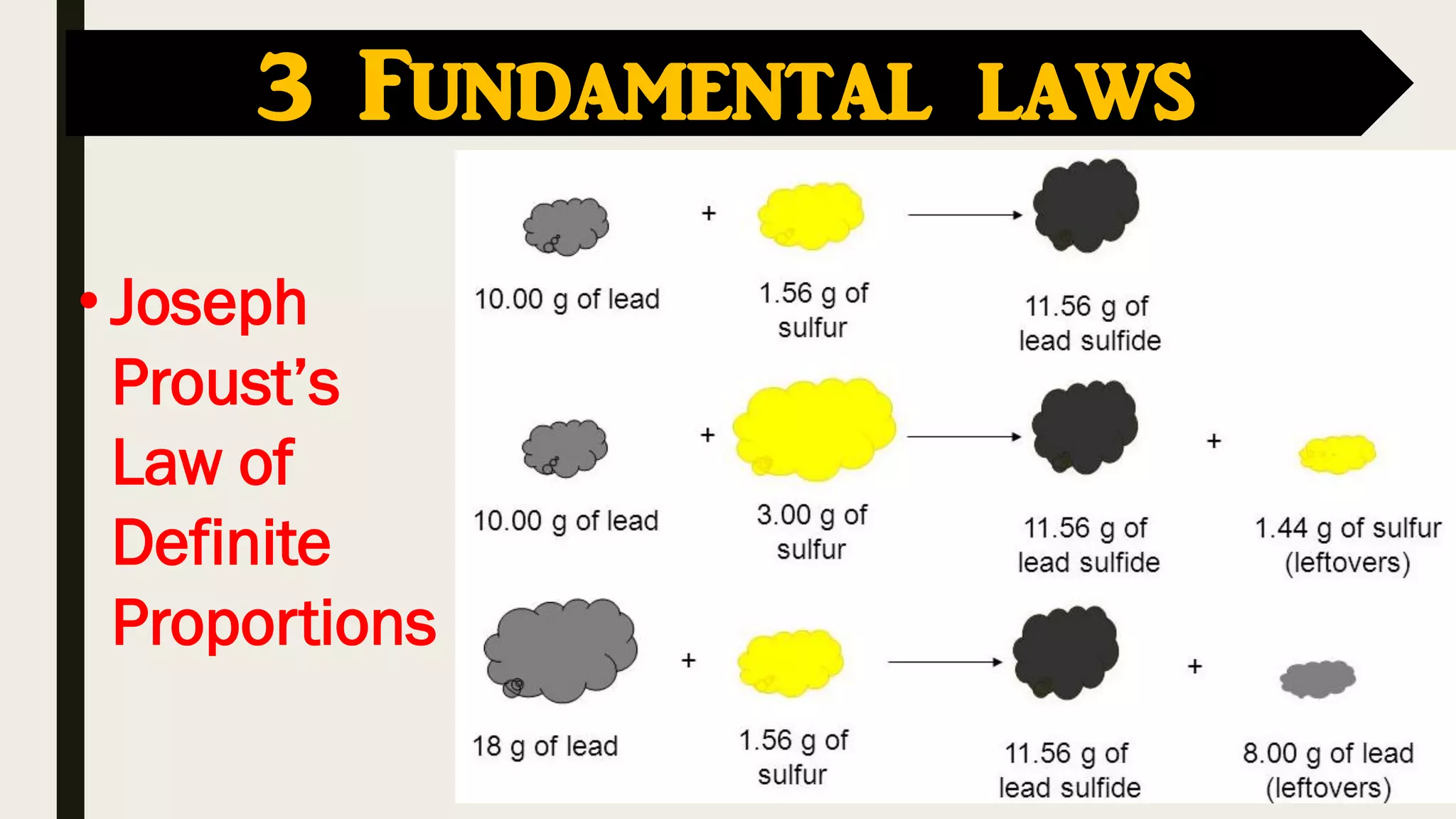
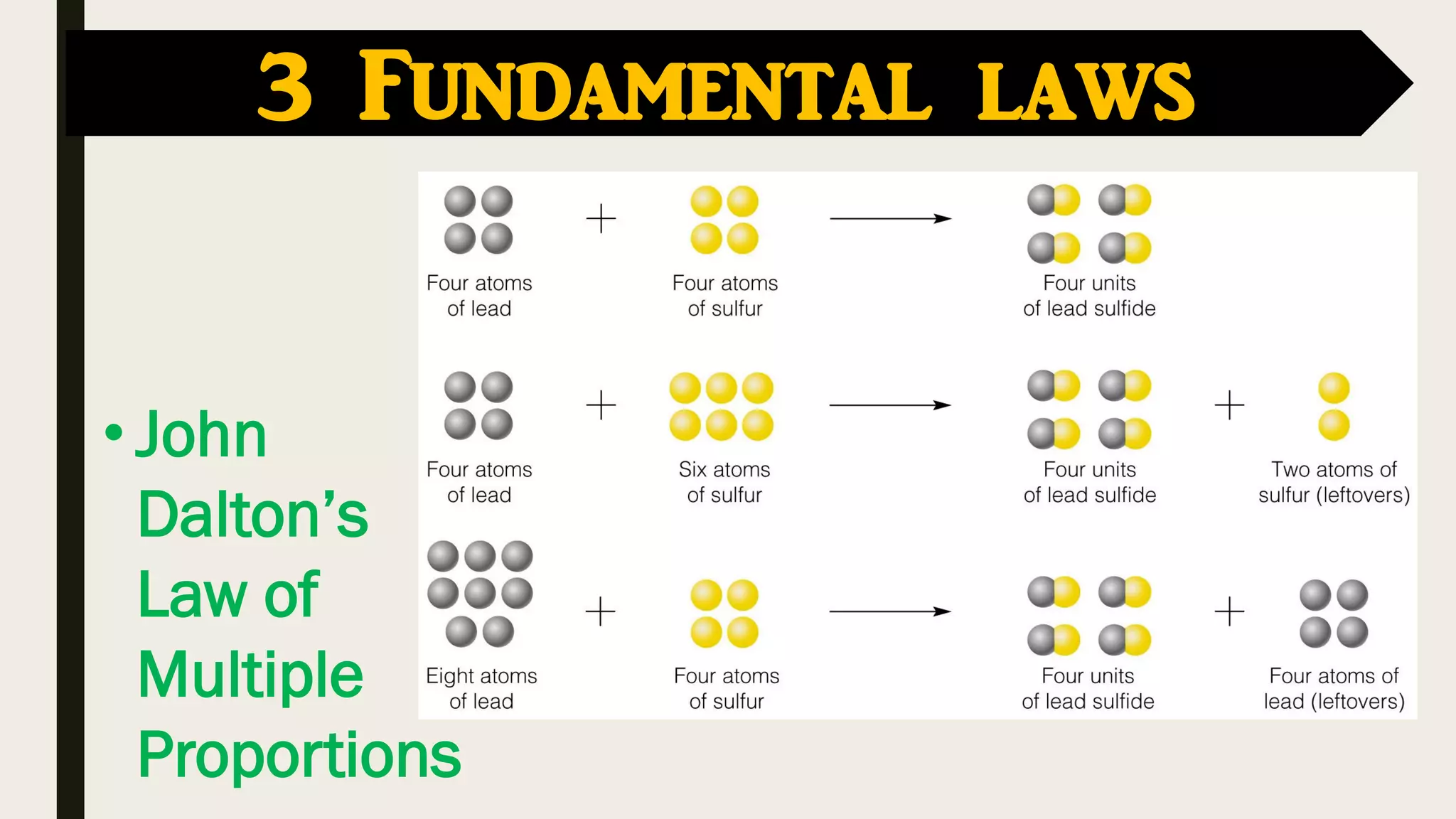
The document covers key historical figures in chemistry, including Robert Boyle, Antoine Lavoisier, John Dalton, Joseph Gay-Lussac, Amedeo Avogadro, and Dmitri Mendeleev, detailing their contributions to the understanding of elements, compounds, and atomic theory. Specific fundamental laws such as the conservation of mass, definite proportions, and multiple proportions are highlighted. It concludes with Mendeleev's development of the periodic table, which organized elements by atomic weight and predicted the existence of undiscovered elements.