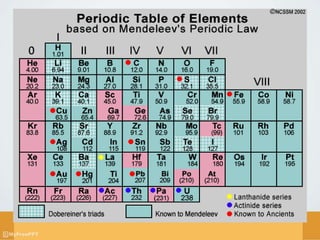
Chemistry is the branch of science concerned with the substances that matter is composed of, their properties, and how they interact or combine. The document provides a brief history of chemistry, outlining key figures and discoveries from ancient civilizations through the 20th century that advanced the field. It describes the main branches of chemistry - organic, analytical, physical, inorganic, and biochemistry - and provides short definitions of each.