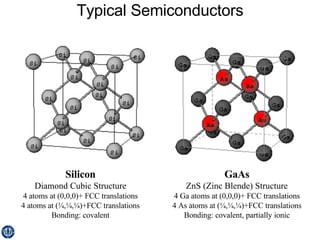
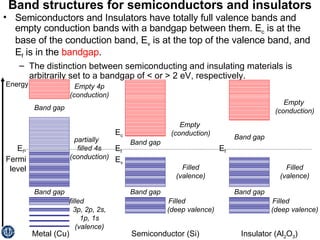
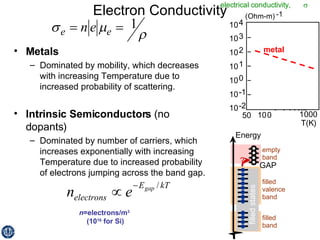
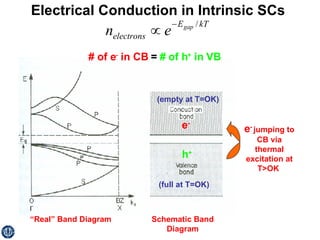
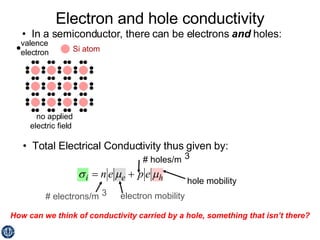
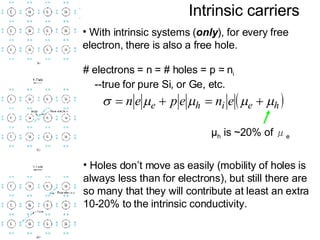
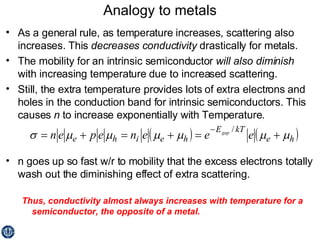
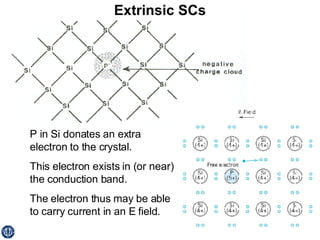
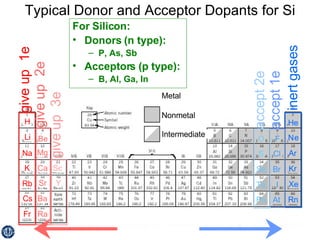
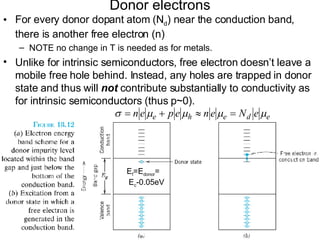
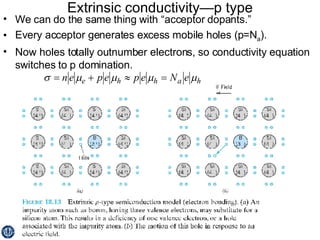
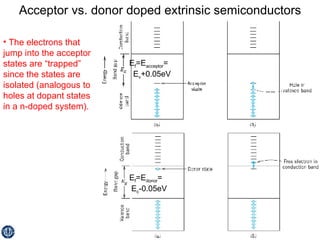
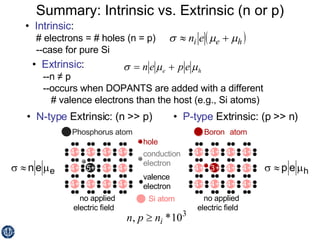
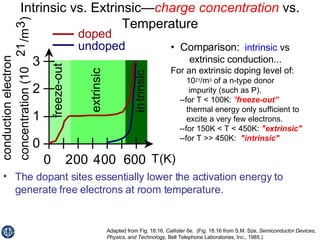
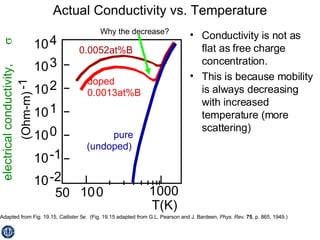
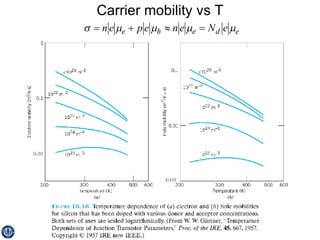
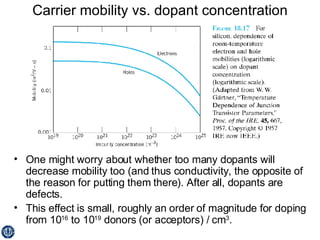
This document discusses semiconductors and integrated circuits. It explains how band diagrams differ for metals, insulators, intrinsic semiconductors and n-type and p-type doped semiconductors. Conductivity increases with temperature for intrinsic semiconductors as carrier concentration rises exponentially. For extrinsic semiconductors, carrier concentration is independent of temperature. Common n-type dopants for silicon include phosphorus, arsenic and antimony, while common p-type dopants include boron, aluminum and gallium. The conductivity equations differ for intrinsic, n-type and p-type materials depending on majority carrier. Carrier concentration changes with temperature and doping level for intrinsic and extrinsic semicon