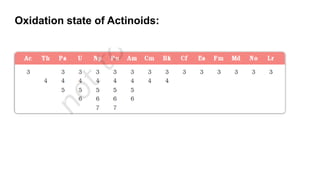
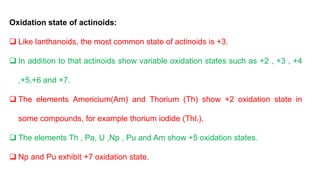
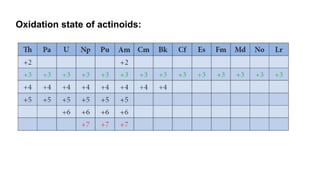
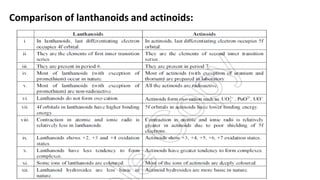
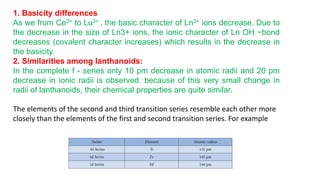
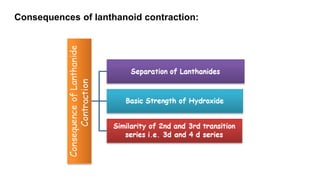
The document provides an overview of f-block elements, specifically lanthanides and actinides, detailing their electronic configurations, oxidation states, and properties. Lanthanides, characterized by a common +3 oxidation state and a gradual lanthanoid contraction, show decreasing ionic and atomic radii across the series, affecting their chemical behavior and separation. Actinides, with more variable oxidation states, are primarily recognized for their radioactive properties and applications in energy generation and nuclear technology.




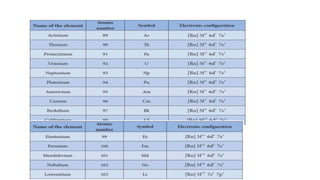
![Electronic configuration:
•Lanthanides: [Xe]4f1–145d0–16s2](https://image.slidesharecdn.com/f-blockelements-200418075745/85/f-block-elements-5-320.jpg)
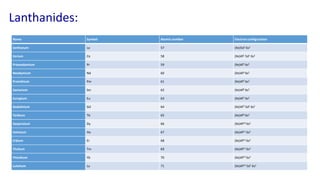






![Actinides: In actinides the differentiating electron enters 5f orbitals.
These are thorium to lawrencium. These elements come immediately
after actinium.
•Actinides: [Rn]5f1–146d0–17s2
Electronic configuration:](https://image.slidesharecdn.com/f-blockelements-200418075745/85/f-block-elements-16-320.jpg)