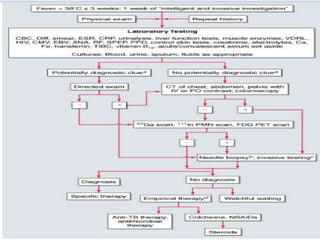
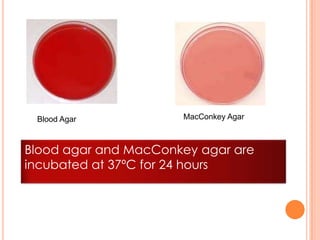
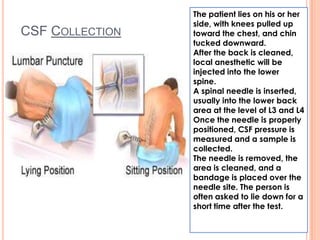
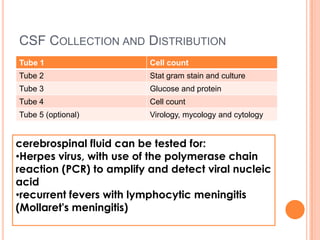
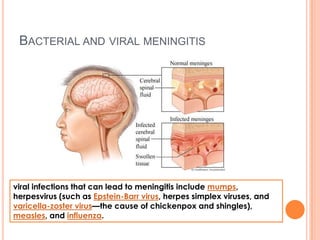
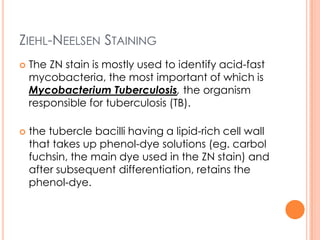
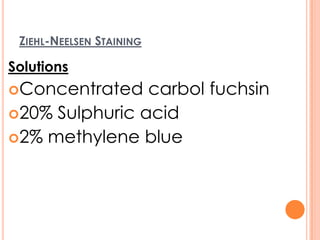
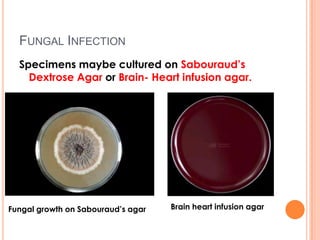
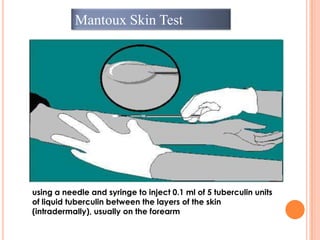
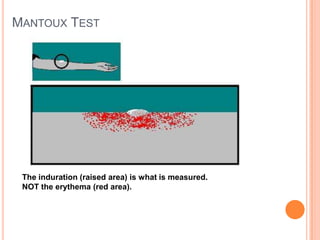
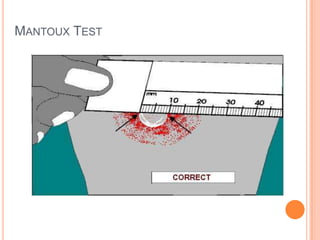
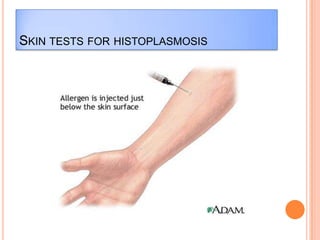
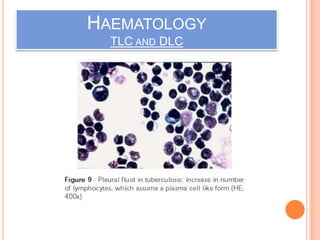
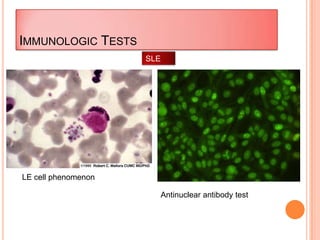
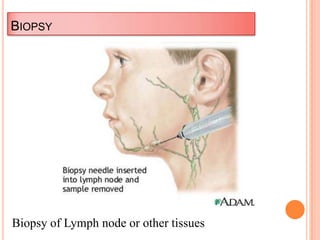
This document outlines the diagnostic approach and laboratory tests for evaluating a patient presenting with pyrexia of unknown origin (PUO). It describes collecting relevant clinical history and performing a physical exam. Specimens including blood, urine, sputum, CSF and tissues may be obtained for bacterial, viral, parasitic and fungal cultures and stains. Tests like blood cultures, urine cultures, sputum smears and cultures, and CSF analysis can help identify potential infectious causes. Serology, skin tests, hematology, immunology and biopsy may also provide diagnostic clues. Empiric antibiotic therapy is guided by risk factors and test results.