Recommended
PPTX
Microscopy_ZN_BASICS_OF_NTEP_AKA_2025.pptx
PPTX
Sputum examination cytology and microscopy
PPTX
DOCX
Detailed examination of tuberculosis in microbiology laboratory including zn ...
PPTX
Ziehl–Neelsen staining for medical students
PPTX
PPTX
Diagnosis of different kinds of Mycobacteriology.pptx
PPTX
Diagnosis of Mycobacteriology and others.pptx
PPT
Lab Diagnosis of Bacterial infections
PPTX
Session 06 Ziehl Neelsen.pptx wealth examinaton
PPT
this presentation is about bacteria staining.ppt
PDF
6-laboratorydiagnosisofbacterialinfection-150727150744-lva1-app6892.pdf
PDF
6-laboratorydiagnosisofbacterialinfection.pdf
PPT
6 laboratory diagnosis of bacterial infection
PPTX
Presentation sputum....by gloria asantewaa
PPTX
Laboratory diagnosis of PUO
PPT
2- Diagnosis of inf. and staining.ppt
PPT
PDF
Medical Microbiology Laboratory (Mycobacterium spp.)
PDF
Mycobacterium tuberculosis (Practical Medical Microbiology, 14)
PPT
Mycobacteriology Update 2018
PPTX
sputum analysis. types of tests to diagnose sputum nature
PPT
PPTX
sputum examination pathology 1st year Allied health Sciences RGUHS.pptx
PPTX
afbstainbymanoj-17053019093Manoj mahato2.pptx
PPTX
Conventional lab diagnosis of tb
PPTX
Presentation of laboratory diagnosis of tb final research
PPTX
Laboratory diagnosis of tuberculosis pract.
PPTX
Hemoglobin_in_Laboratory_Presentation.pptx
PPTX
Typical_Virus_Like_Agents_Presentation.pptx
More Related Content
PPTX
Microscopy_ZN_BASICS_OF_NTEP_AKA_2025.pptx
PPTX
Sputum examination cytology and microscopy
PPTX
DOCX
Detailed examination of tuberculosis in microbiology laboratory including zn ...
PPTX
Ziehl–Neelsen staining for medical students
PPTX
PPTX
Diagnosis of different kinds of Mycobacteriology.pptx
PPTX
Diagnosis of Mycobacteriology and others.pptx
Similar to Sputum_Staining_Guidelines presentations
PPT
Lab Diagnosis of Bacterial infections
PPTX
Session 06 Ziehl Neelsen.pptx wealth examinaton
PPT
this presentation is about bacteria staining.ppt
PDF
6-laboratorydiagnosisofbacterialinfection-150727150744-lva1-app6892.pdf
PDF
6-laboratorydiagnosisofbacterialinfection.pdf
PPT
6 laboratory diagnosis of bacterial infection
PPTX
Presentation sputum....by gloria asantewaa
PPTX
Laboratory diagnosis of PUO
PPT
2- Diagnosis of inf. and staining.ppt
PPT
PDF
Medical Microbiology Laboratory (Mycobacterium spp.)
PDF
Mycobacterium tuberculosis (Practical Medical Microbiology, 14)
PPT
Mycobacteriology Update 2018
PPTX
sputum analysis. types of tests to diagnose sputum nature
PPT
PPTX
sputum examination pathology 1st year Allied health Sciences RGUHS.pptx
PPTX
afbstainbymanoj-17053019093Manoj mahato2.pptx
PPTX
Conventional lab diagnosis of tb
PPTX
Presentation of laboratory diagnosis of tb final research
PPTX
Laboratory diagnosis of tuberculosis pract.
More from masrrataha
PPTX
Hemoglobin_in_Laboratory_Presentation.pptx
PPTX
Typical_Virus_Like_Agents_Presentation.pptx
PPTX
sterilization & Disinfection of instruments
PPTX
Cross infection in medical microbiology.pptx
PPTX
bacterial growth microbiology presentation
PPTX
Antibiotic Policies and stewardship.pptx
Recently uploaded
PDF
Key Pharmaceutical Advisory Firms for Early-Stage Biotech Businesses
PPTX
Acute kidney injury and its management.pptx
PPTX
Tonsillitis tonsillitis tonsillitis tonsillitis tonsillitis tonsillitis
PDF
SEMINARIO BIOMOL.pdf (Samuel Cantillo y Karen Bohorquez)
PPTX
ACC+HBP+Clinical+Highlights+Slide+Deck_Final.pptx
PDF
1150130-高雄地區第517次小兒科聯合病例討論會--高雄市醫師公會.pdf
PPTX
Management of diarrhea (medical & Nursing).pptx
PDF
Bias in Clinical Trials - Dr Jigar Savaliya.pdf
PPTX
A War on Heart Attacks -- Joel Kahn, MD, FACC
PPTX
1.3 inflamation, introduction to inflamation by Dr Lelaka.pptx
PPTX
chronic bronchitis.pptx By gokulakrishnan
PPTX
Approach to Global Developmental Delay (Lecture notes for MBBS)
PDF
Chronic Suppurative Otitis Media: A Comprehensive Review of Epidemiology, Pat...
PPT
PNEUMONIA presentations, and treatment lll
PPTX
nderstanding Male Sexual Health: Challenges and Solutions
PPTX
Formulation Development & Evaluation of Polyherbal Soap
PPTX
HIV.pptx treatment and prophylaxis explained
PPTX
Sliding Filament Theory | Muscle Physiology | KEMU
PPTX
BRONCHIECTASIS.cardio pptx by gokulakrishnan
PDF
Smoking Impact Diagnostic Panel: Understanding Smoking's Hidden Impact on You...
Sputum_Staining_Guidelines presentations 1. 2. Objectives
• - Understand the purpose of sputum staining
• - Learn types of sputum stains
• - Know proper collection and preparation
techniques
• - Identify common findings in stained samples
3. Indications for Sputum Staining
• - Suspected pulmonary infection (e.g., TB,
pneumonia)
• - Monitoring known infections
• - Identifying causative organisms
• - Diagnostic workup of chronic cough
4. Types of Sputum Stains
• - Gram stain
• - Ziehl-Neelsen stain (Acid-Fast Bacilli)
• - Auramine-rhodamine stain (fluorescent)
• - Giemsa stain (for parasites, Pneumocystis)
5. Sputum Sample Collection
• - Collected early morning before eating or
drinking
• - Rinse mouth with water beforehand
• - Instruct patient to take deep cough from
lungs
• - Use sterile, leak-proof container
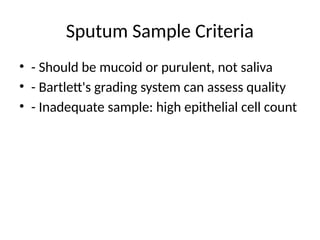
6. Sputum Sample Criteria
• - Should be mucoid or purulent, not saliva
• - Bartlett's grading system can assess quality
• - Inadequate sample: high epithelial cell count
7. Gram Staining Technique
• - Fix smear by heat
• - Apply crystal violet (1 min), rinse
• - Apply iodine (1 min), rinse
• - Decolorize with alcohol (10–30 sec), rinse
• - Counterstain with safranin (1 min), rinse
• - Examine under microscope
8. Ziehl-Neelsen (ZN) Stain
• - Used for Mycobacterium tuberculosis
• - Carbol fuchsin (heat to steam), rinse
• - Decolorize with acid-alcohol, rinse
• - Counterstain with methylene blue, rinse
• - Acid-fast bacilli appear red on blue
background
9. Auramine-Rhodamine Stain
• - Fluorescent stain for AFB
• - Smear is stained with auramine-rhodamine
dye
• - Viewed under fluorescent microscope
• - More sensitive than ZN stain
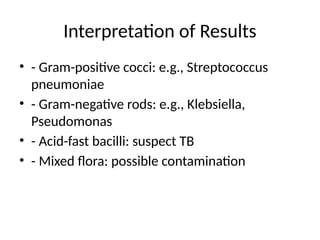
10. Interpretation of Results
• - Gram-positive cocci: e.g., Streptococcus
pneumoniae
• - Gram-negative rods: e.g., Klebsiella,
Pseudomonas
• - Acid-fast bacilli: suspect TB
• - Mixed flora: possible contamination
11. Common Pitfalls
• - Salivary contamination
• - Inadequate staining technique
• - Delayed processing
• - Over-decolorization in AFB staining
12. Summary
• - Proper sample collection is key
• - Choose appropriate stain based on clinical
suspicion
• - Interpret with clinical correlation
• - Staining is essential for diagnosis and
treatment
13. References
• - WHO TB Laboratory Manual
• - Bailey & Scott's Diagnostic Microbiology
• - CDC Laboratory Methods
• - Medical Microbiology textbooks
![Sputum Staining Guidelines
• A Guide for Medical Students
• Presented by: [Your Name/Institution]
• Date](https://image.slidesharecdn.com/sputumstainingguidelines-250613093845-fee71cda/75/Sputum_Staining_Guidelines-presentations-1-2048.jpg)