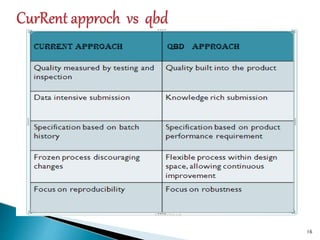
This document introduces the key concepts of Quality by Design (QbD), including:
1) QbD ensures product quality is built in from the beginning through understanding how formulation and manufacturing processes impact quality.
2) Critical Quality Attributes and Critical Process Parameters are identified based on a Target Product Quality Profile to ensure desired product quality.
3) Risk assessment determines which factors most influence quality and a design space is established where quality can be assured.
4) A control strategy is developed to evaluate and ensure quality through all stages of development and manufacturing.







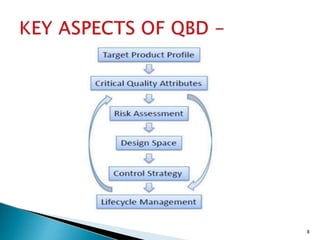
![1] The Target Product Quality Profile (TPQP) -
9
TPQP has been defined as a “prospective and dynamic
summary of the quality characteristics of a drug product that
ideally will be achieved to ensure that the desired quality, and thus
the safety and efficacy, of a drug product is realized”.
⦁ TPP forms the basis for product design in the following way
Dosage form, Route of administration, Strength, Release
Pharmacological characteristic
Drug product quality criteria
Pharmaceutical elegance](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-9-320.jpg)
![2] Critical Quality Attribute (CQA) -
⦁ Once TPQP has been identified, the next step is to identify the
relevant CQAs.
⦁ A CQA has been defined as “a physical, chemical, biological, or
microbiological property or characteristic that should be within an
appropriate limit, range, or distributed to ensure the desired
product quality”
⦁ Prior product knowledge, such as the accumulated laboratory,
nonclinical and clinical experience with a specific product-
quality attribute, is the key in making these risk assessments.
10](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-10-320.jpg)
![3] Critical Process Parameter (CPPs) -
⦁ Critical process parameters (CPPs) are defined as “parameters
whose variability have an impact on a CQA and therefore should
be monitored or controlled to ensure the process produces the
desired quality”
⦁ Process robustness is defined as the ability of a process to
demonstrate acceptable quality and performance and tolerate
variability in inputs at the same time.
11](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-11-320.jpg)
![4] Risk Assessment
⦁ Quality risk management is a systematic process for the
assessment, control, communication and review of risks to the
quality of the drug (medicinal) product across the product
lifecycle.
⦁ The initial list of potential parameters which can affect CQAs
can be quite extensive but can be reduced by quality risk
assessment (QRA).
12](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-12-320.jpg)
![5] Design Space –
⦁ The ICH Q8(R2) States that the design space is multi
dimensional combination and interaction of input variables (e.g.,
material attributes) and process parameters that have been
demonstrated to provide assurance of quality.
⦁ Working within the design space is not considered as a change.
Movement out of the design space is considered to be a change
and would normally initiate a regulatory post approval change
process .
13](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-13-320.jpg)
![6] Control Strategy-
Control strategy is defined as “a planned set of controls,
derived from current product and process understanding that
assures process performance and product quality”.
The ability to evaluate and ensure the quality of in- process
and/or final product based on process data which typically
include a valid combination of measured material attributes and
process controls. ICH Q8(R2).
el
The control strategy can include the following
elements: procedural controls, in process controls, lot release
testing, process monitoring, characterization testing, comparability
testing and stability testing .
14](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-14-320.jpg)
![7] Life Cycle Management
⦁ In the QBD paradigm, process changes within the design space
will not require review or approval.
⦁ Therefore, process improvements during the product life cycle
with regard to process consistency and throughput could take
place with fewer post approval submissions.
15](https://image.slidesharecdn.com/sathishcadd2-231030161811-3db934d3/85/sathish-cadd-2-pptx-15-320.jpg)