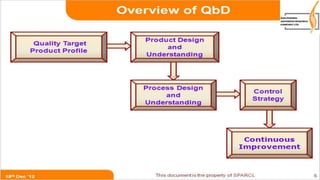
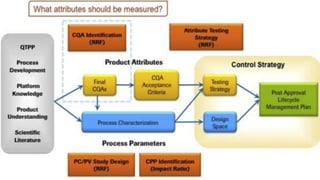
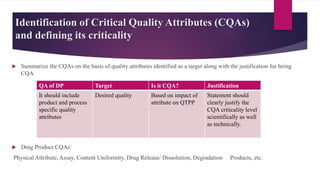
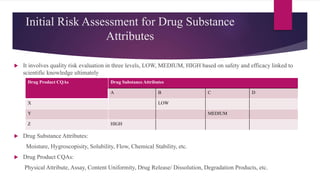
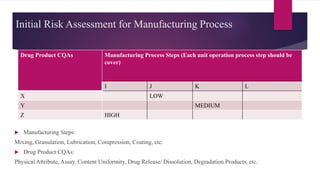
The document provides an overview of quality by design (QbD) in pharmaceutical development. It discusses key QbD concepts like defining a quality target product profile, identifying critical quality attributes and critical material attributes, designing quality into the product through development strategies like design of experiments, and establishing a design space and control strategy. The document outlines the important steps and aspects of a QbD-based pharmaceutical development process from formulation to manufacturing. It emphasizes gaining process understanding, building quality into the product, and taking a systematic risk-based approach.