This document provides an overview of key chemistry concepts including:
1. Chemistry deals with matter and changes in matter at the macroscopic and microscopic levels. The scientific problem solving process involves stating the problem, formulating a hypothesis, and testing it with experiments.
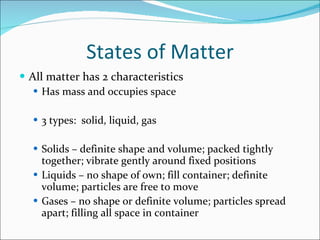
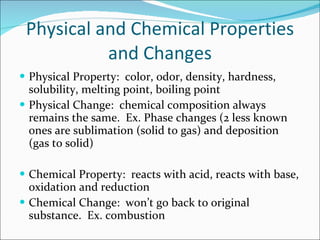
2. The three states of matter are solids, liquids, and gases which differ in their particle arrangements and ability to change shape or volume. Physical and chemical properties and changes also differ based on whether the chemical composition changes.
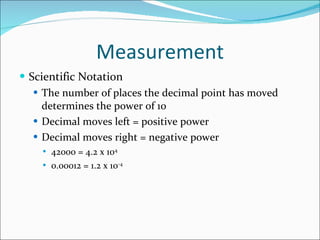
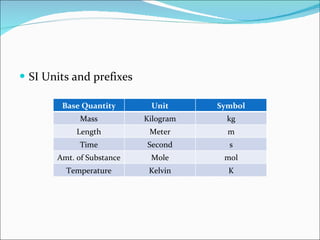
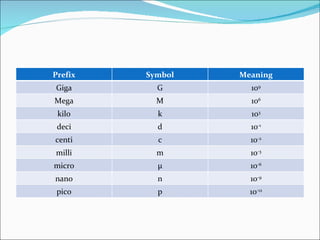
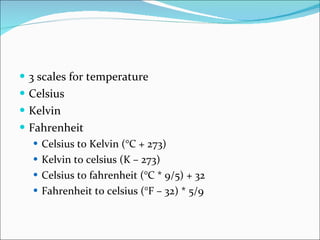
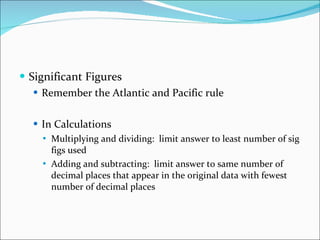
3. Common units and measurements in chemistry include the mole, SI units, scientific notation, uncertainty in measurements, and calculating percent error.