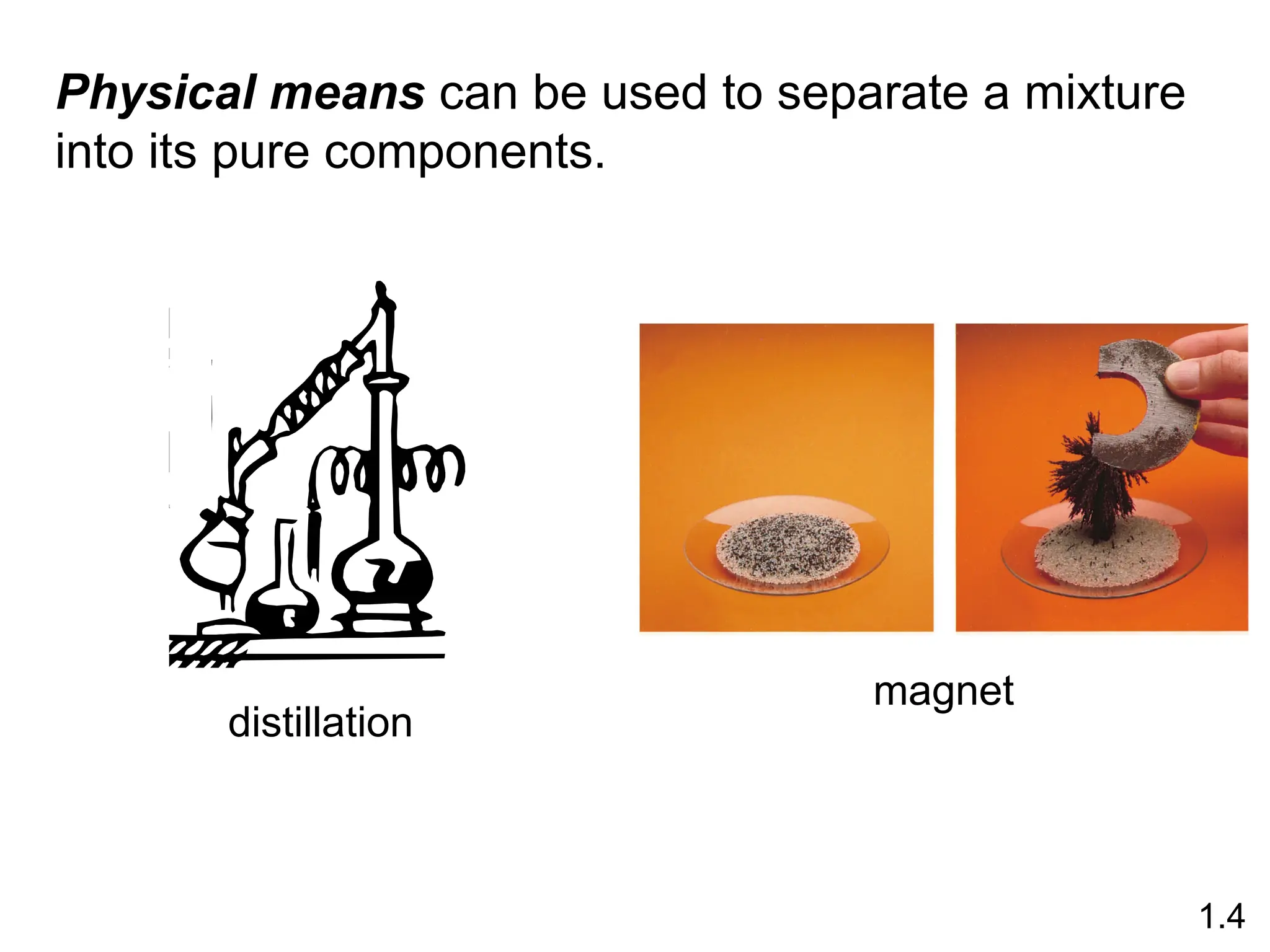
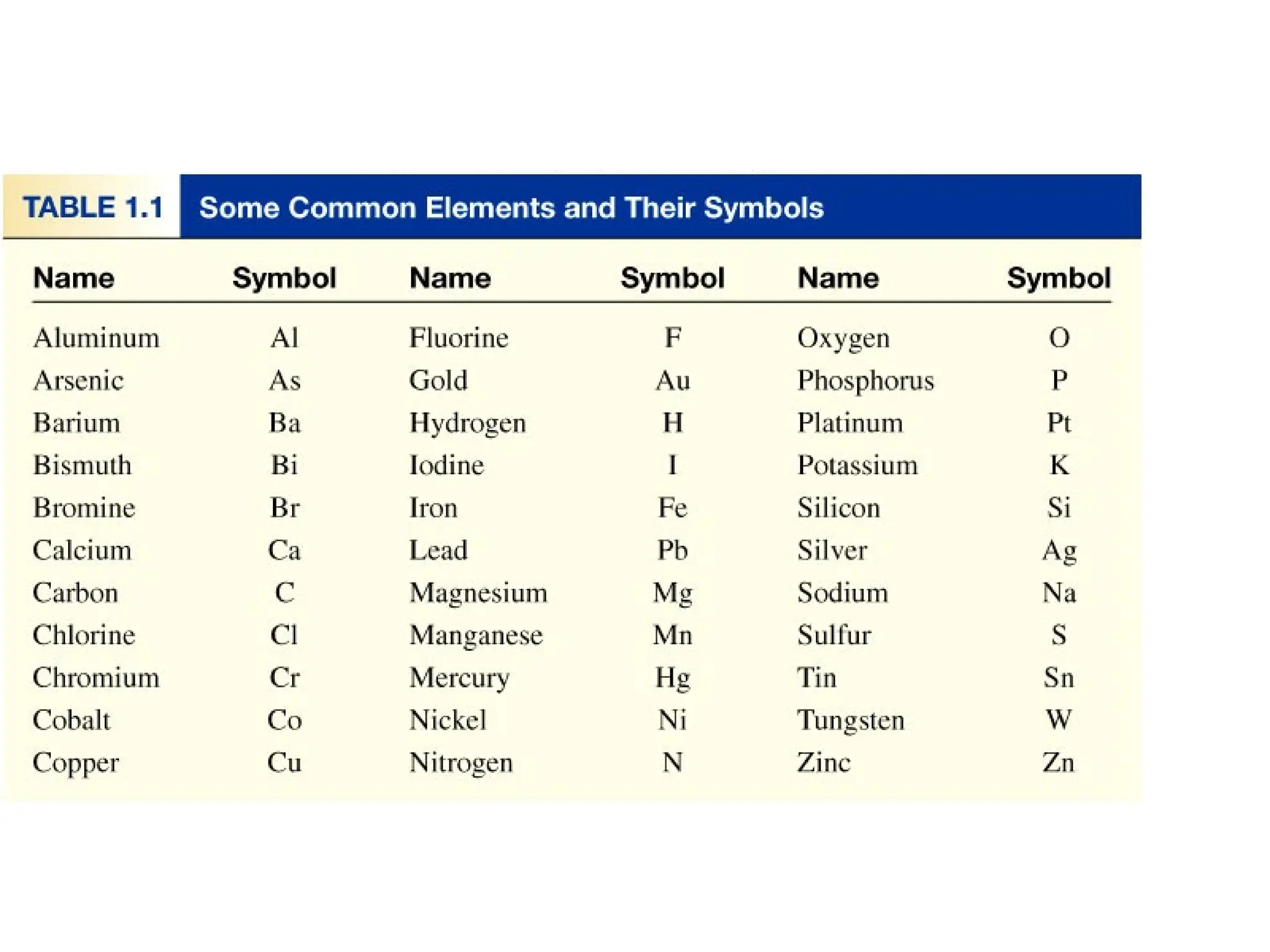
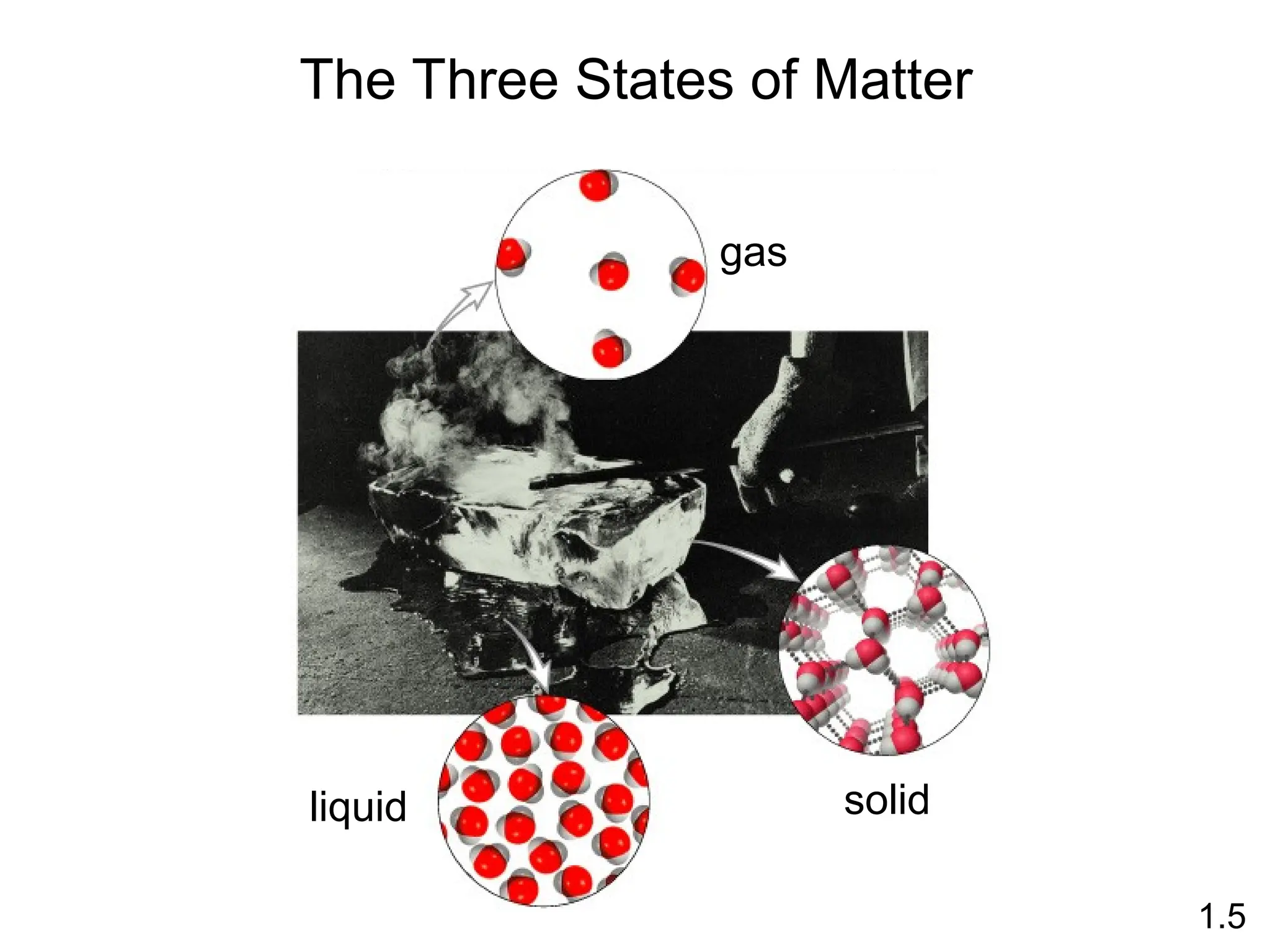
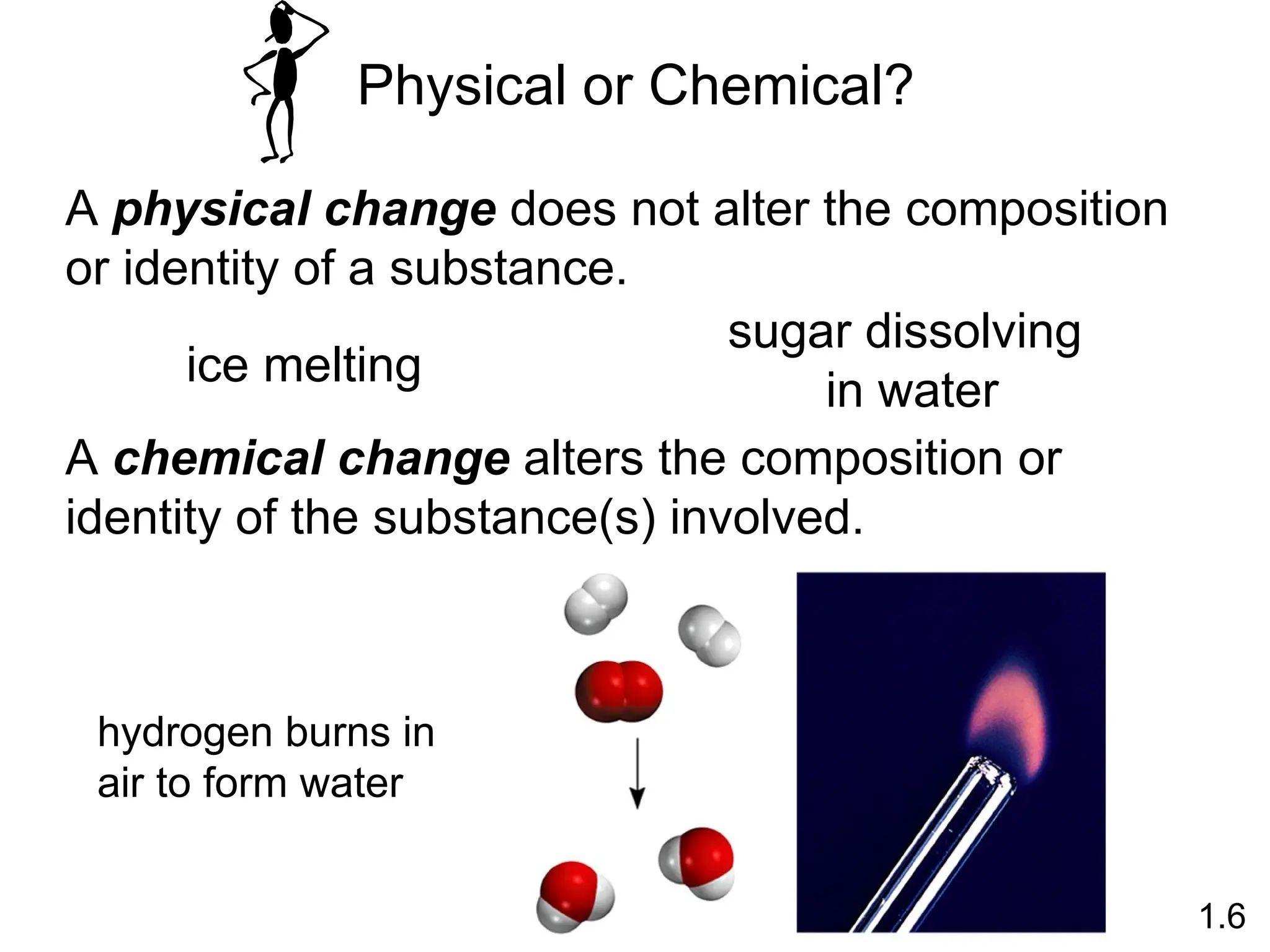
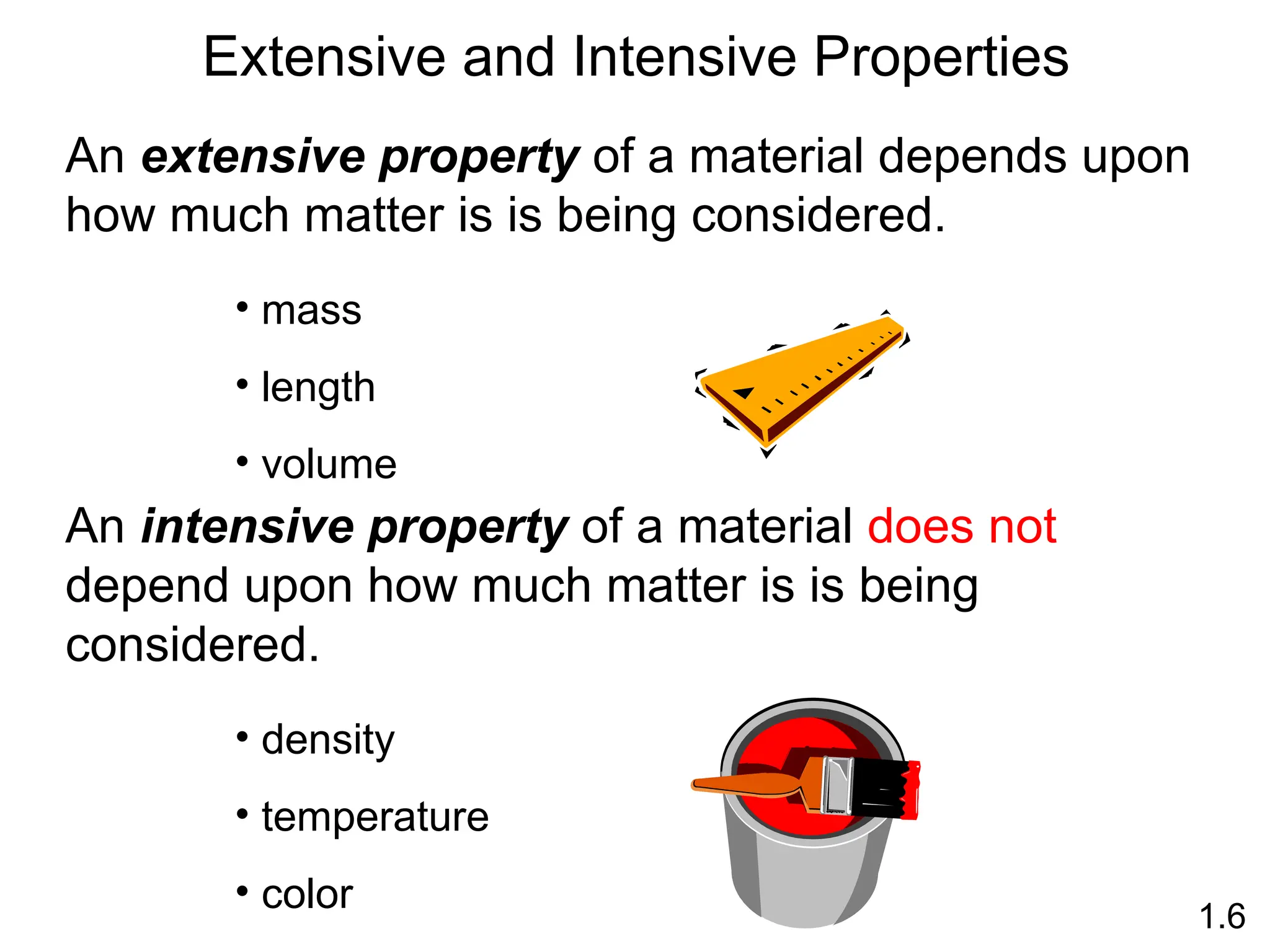
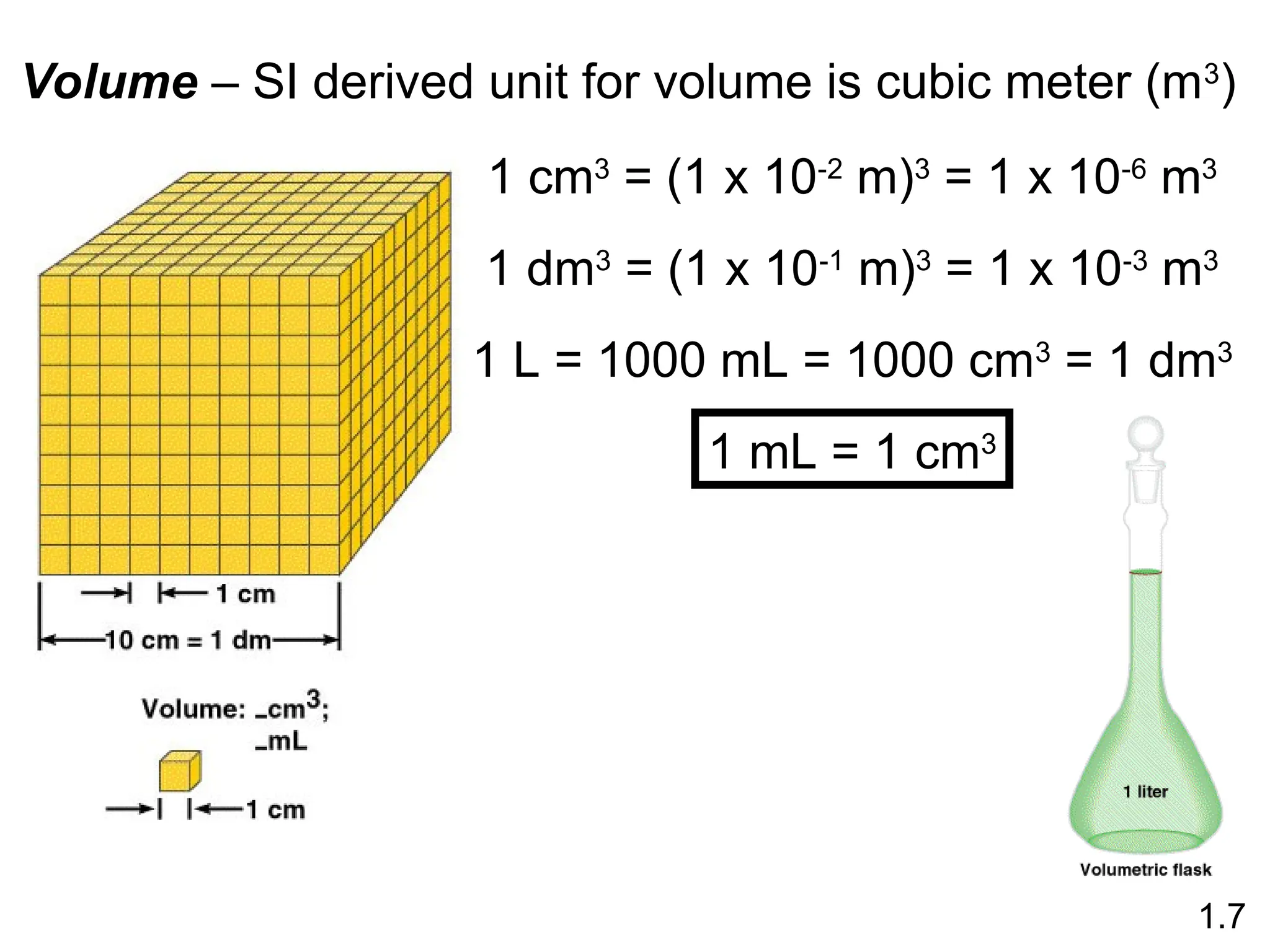
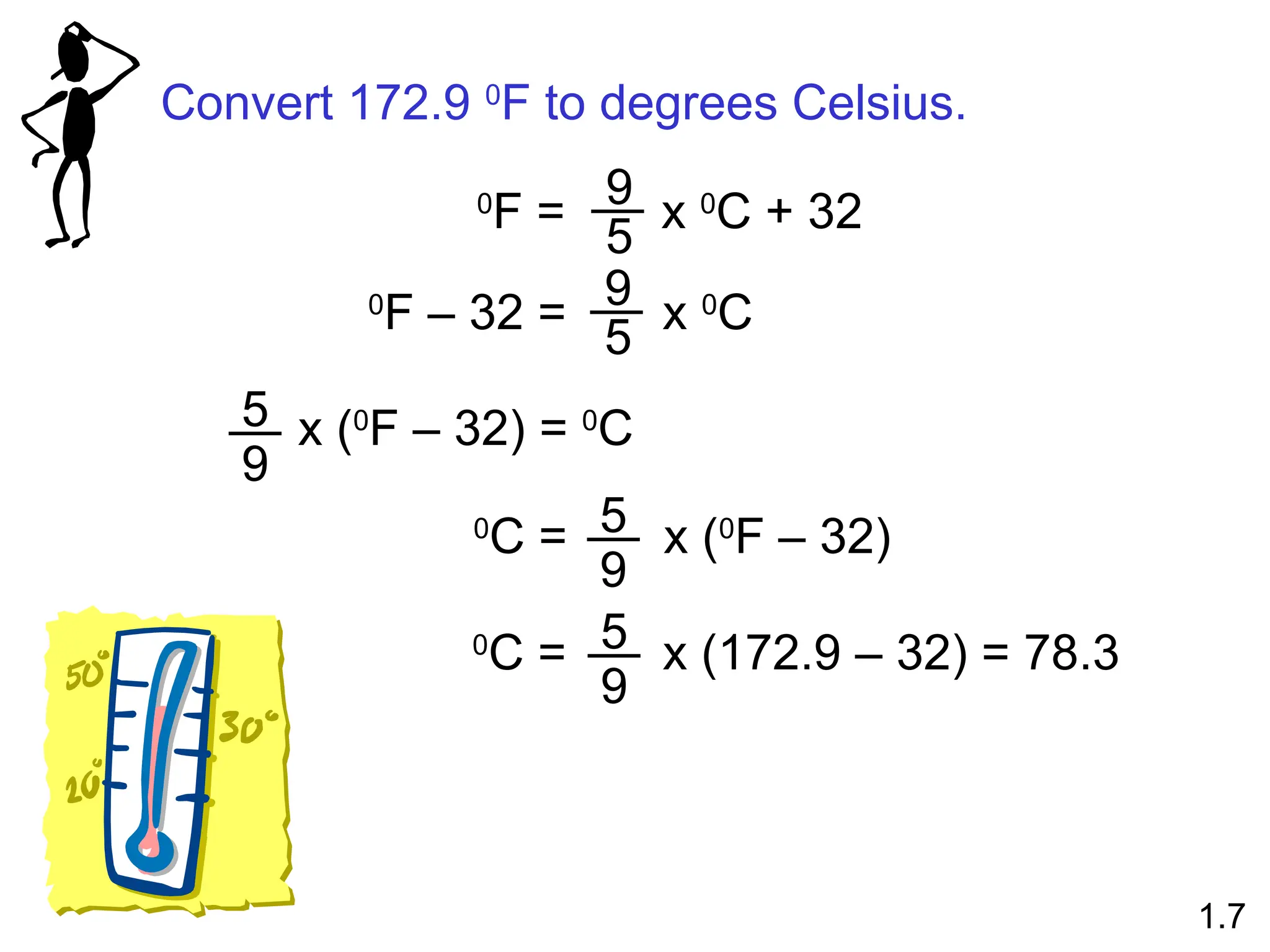
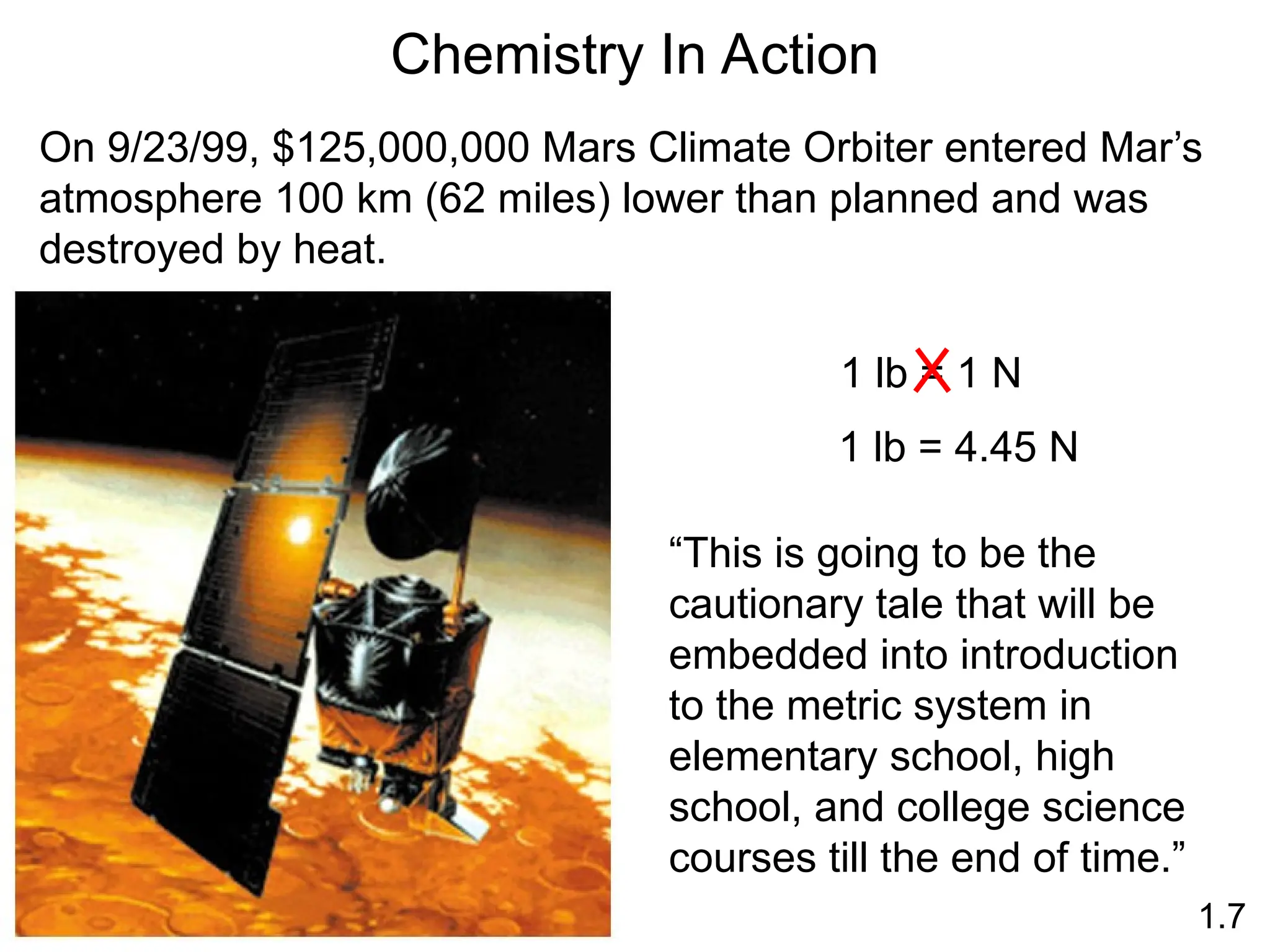
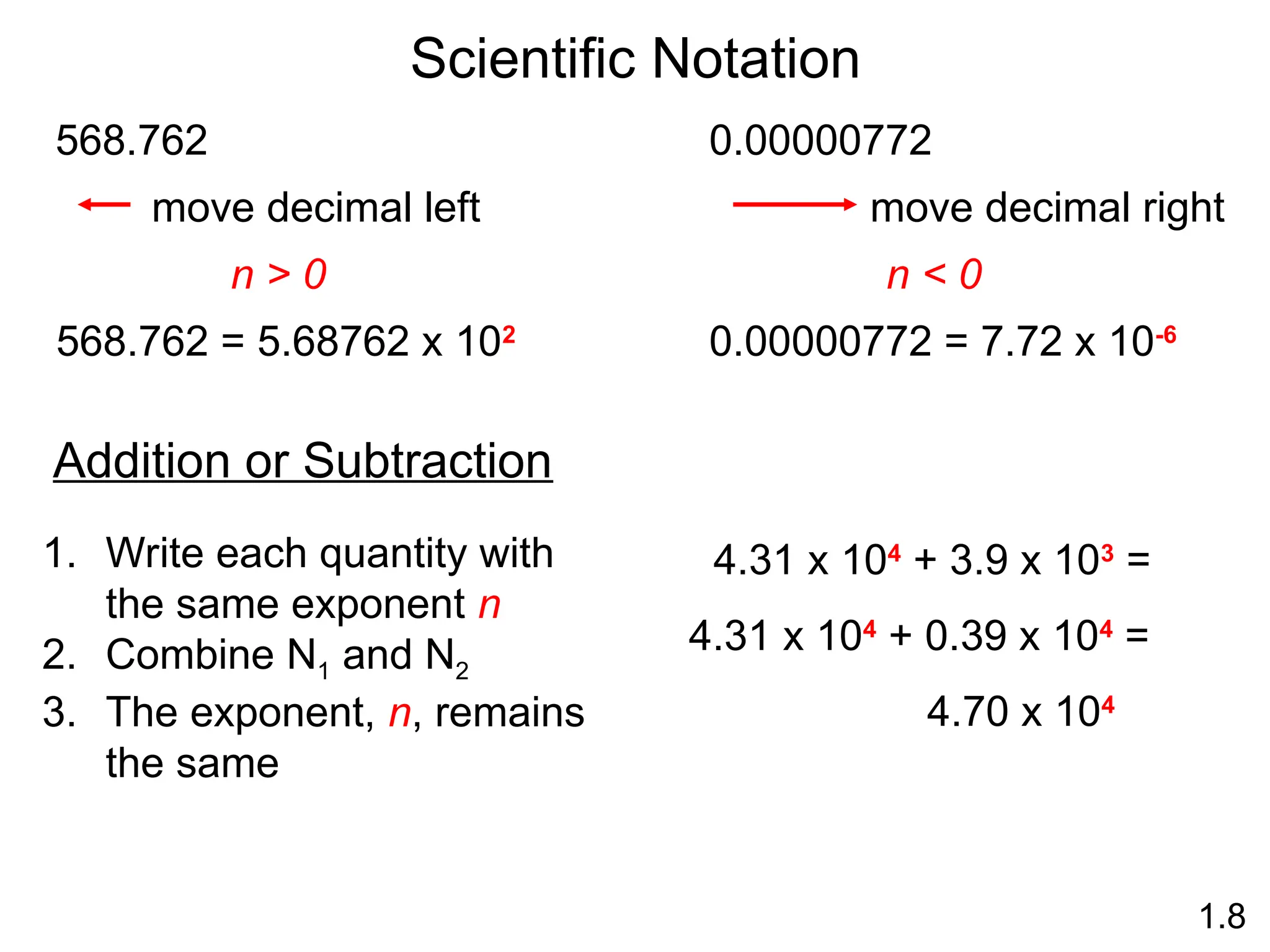
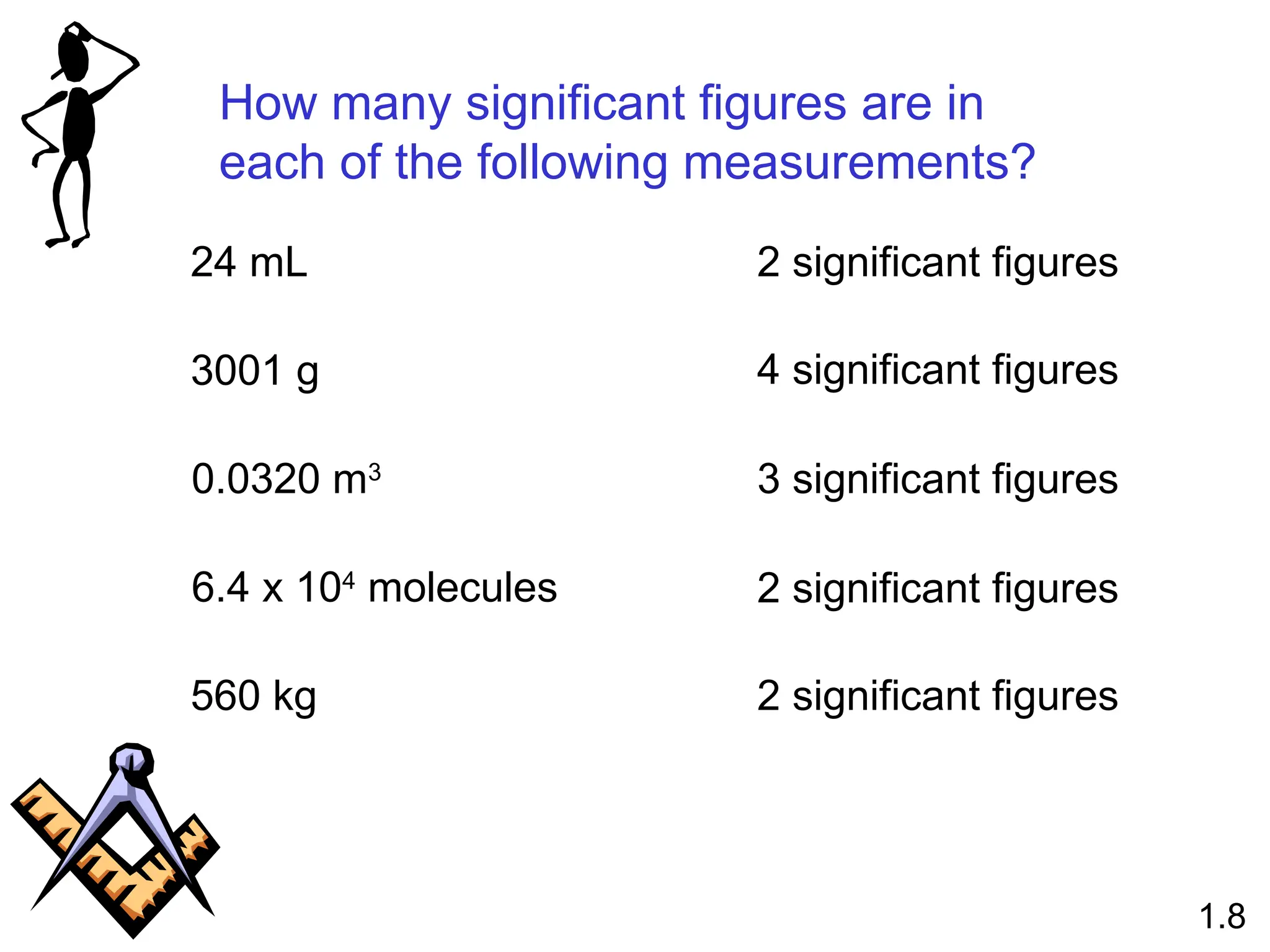
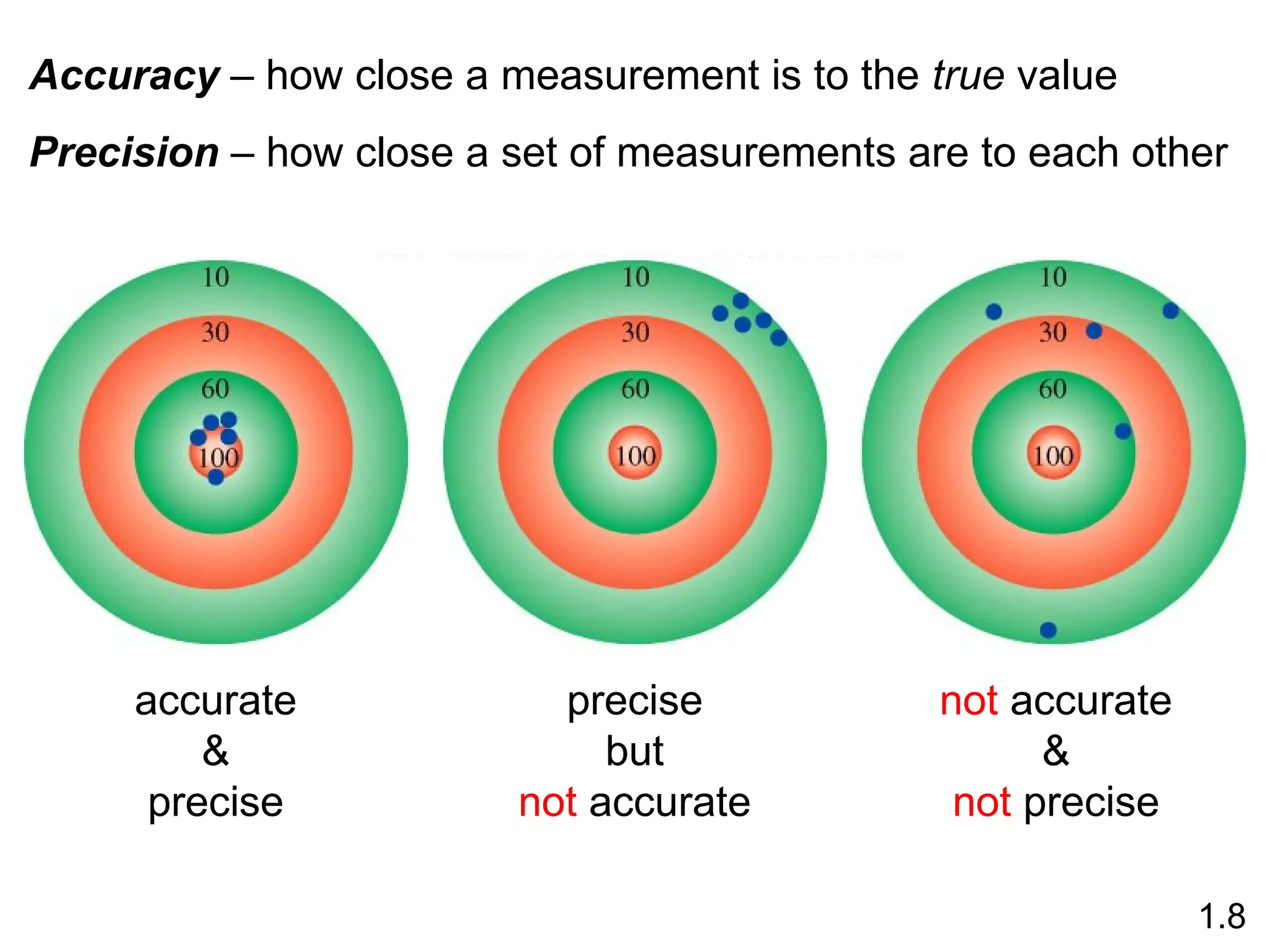
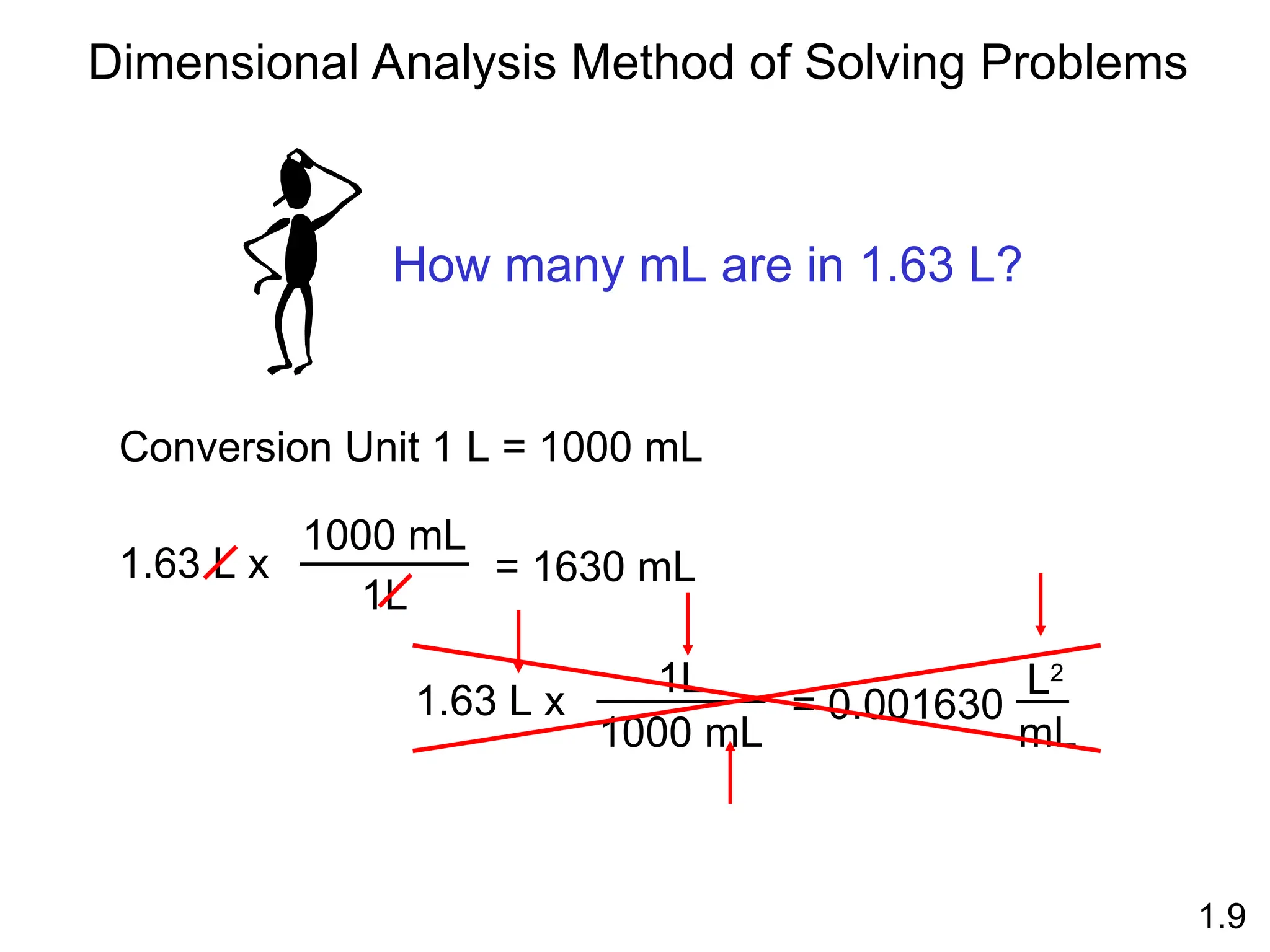
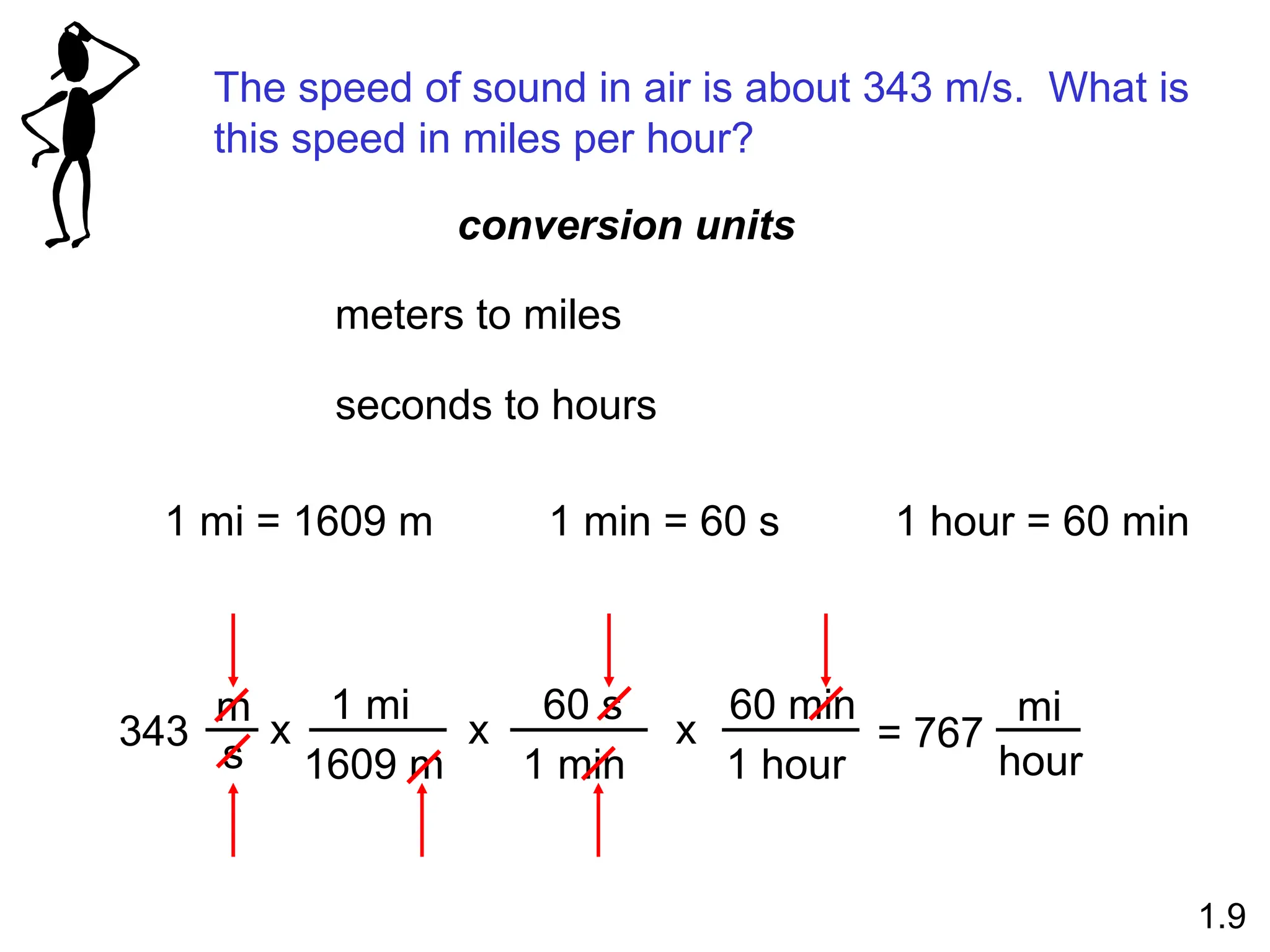
The document provides a comprehensive overview of chemistry, focusing on its definitions, principles, and various applications in modern science, such as health, energy, and technology. It discusses key concepts like the scientific method, classifications of matter, states of matter, and the distinction between physical and chemical changes. Additionally, it covers measurement units, significant figures, and dimensional analysis for problem-solving in a scientific context.