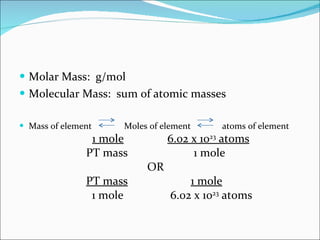
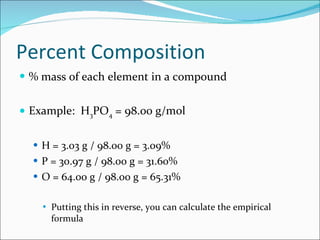
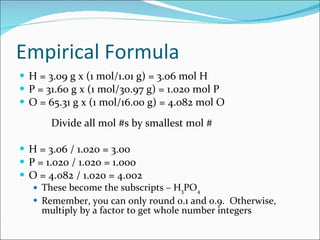
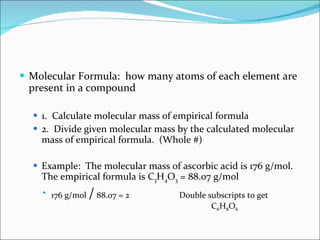
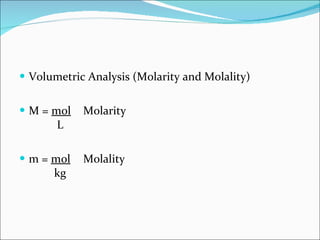
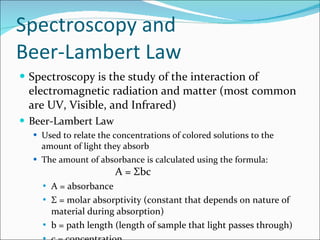
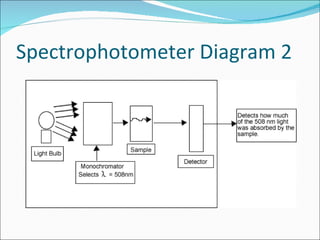
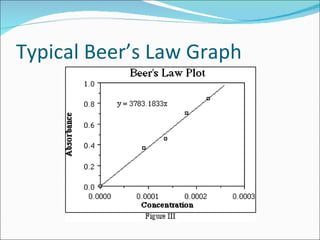
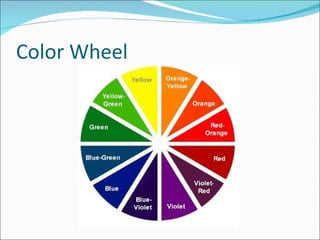
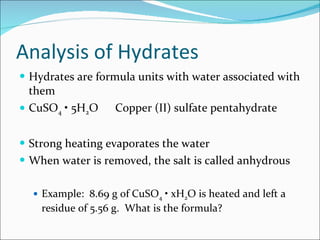
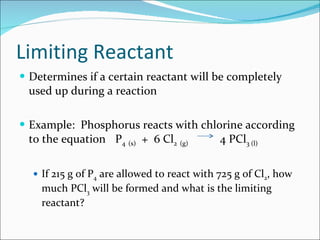
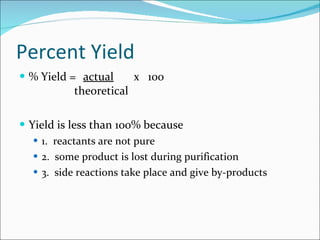
Stoichiometry is the quantitative study of chemical reactions and their mole-based ratios. It allows one to calculate amounts of substances involved in reactions based on molar masses, moles, and balanced chemical equations. Key concepts include empirical and molecular formulas, molarity, dilution calculations, spectrophotometry using Beer's Law, colorimetry, and determining limiting reactants.