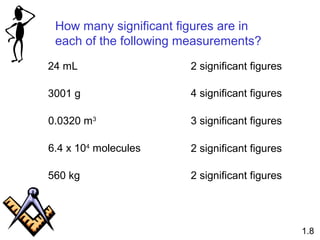
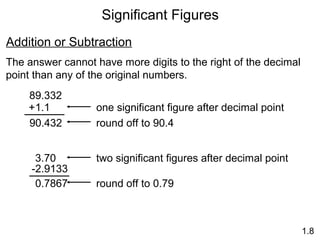
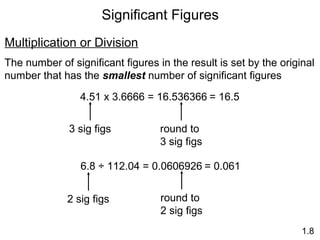
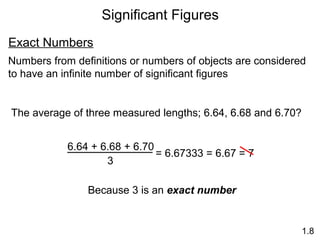
This document provides an overview of chemistry concepts including:
- Chemistry is the study of matter and its properties, covering topics like health, energy, materials, food and more.
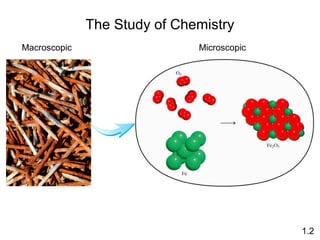
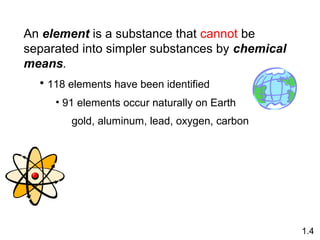
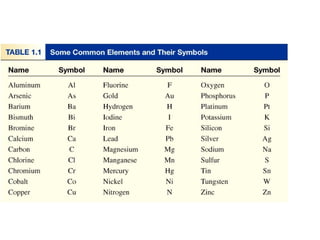
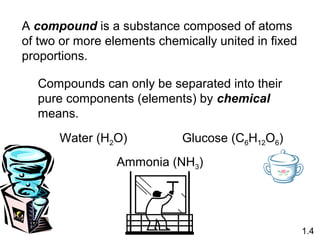
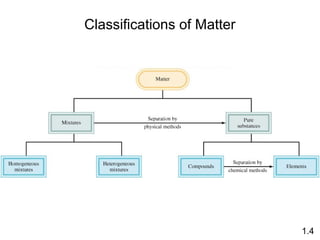
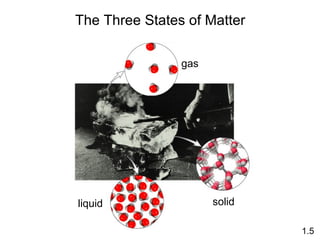
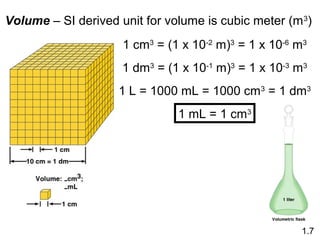
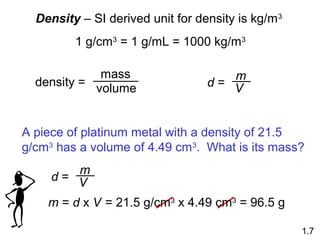
- Matter is anything that has mass and takes up space, and can exist as elements, compounds and mixtures.
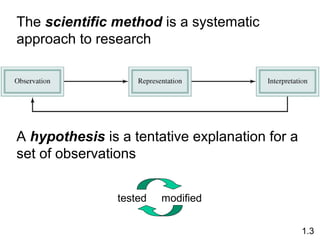
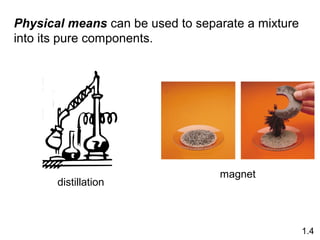
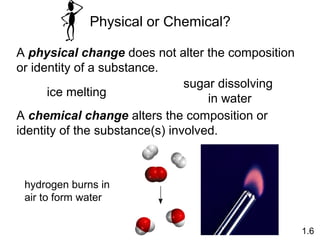
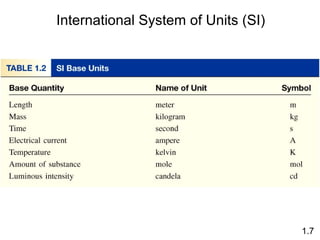
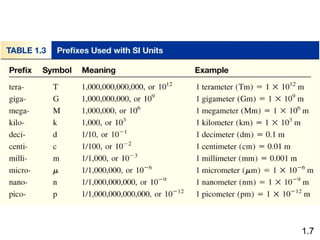
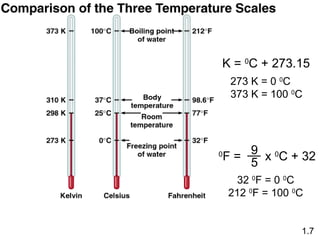
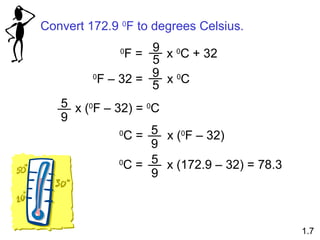
- Chemical and physical changes alter substances in different ways. The document also introduces concepts such as states of matter, properties of matter, units of measurement, and dimensional analysis.