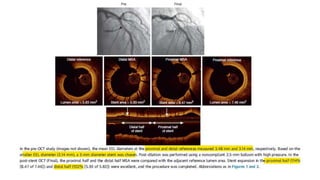
This document discusses the use of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) for assessing coronary artery disease and optimizing percutaneous coronary intervention (PCI). Some key points:
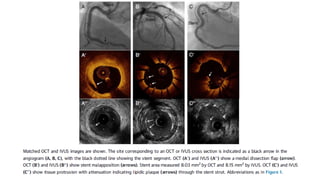
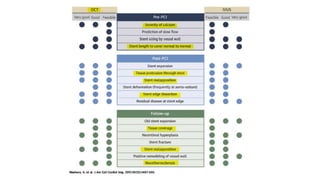
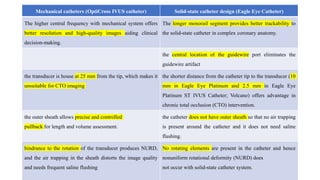
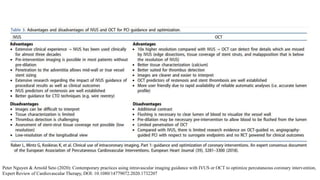
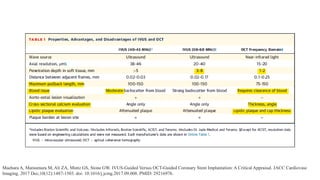
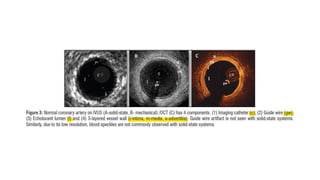
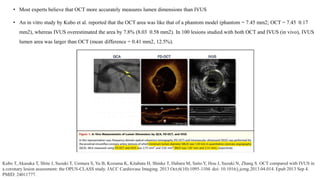
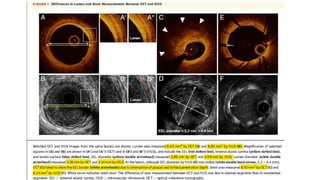
1) IVUS has better tissue penetration than OCT but lower resolution. OCT has much higher resolution which allows more accurate lumen measurement.
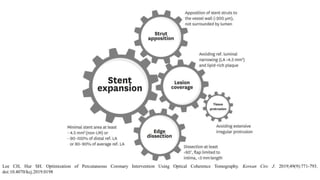
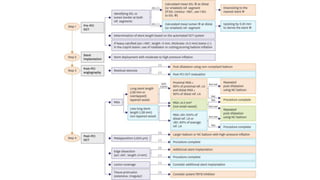
2) Both IVUS and OCT can help optimize stent implantation by informing lesion preparation, stent sizing and placement to minimize geographic miss and under expansion.
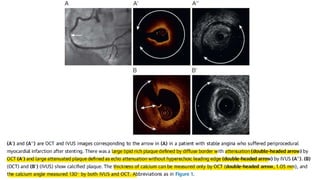
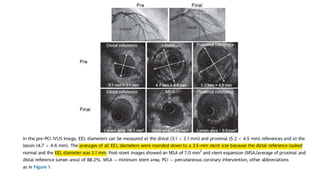
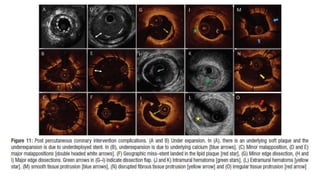
3) Post-PCI, a minimum stent area (MSA) below 5mm2 seen on IVUS/OCT is associated with higher risk of restenosis and stent thrombosis. Under expansion is still common.









![PRE-INTERVENTION EVALUATION OF PLAQUE TYPE RELATED TO
ACUTE STENT OUTCOMES
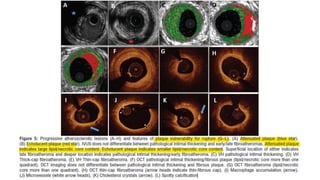
• LIPIDIC PLAQUE AND DISTAL EMBOLIZATION.
• Baseline IVUS or OCT may predict distal embolization and subsequent periprocedural myocardial infarction (MI) after PCI
in native arteries and re-stenotic lesions
• Morphological predictors of periprocedural MI in observational studies are
1. attenuated plaque (indicating a large necrotic core) or
2. plaque rupture by IVUS,
3. necrotic core by virtual histology IVUS, and
4. thin-cap fibroatheroma or plaque rupture by OCT.
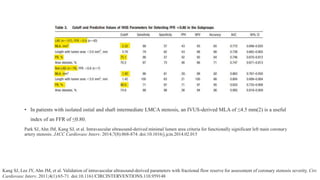
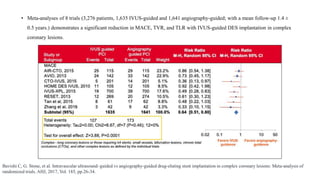
• In a large IVUS study of 336 patients with acute coronary syndrome (ACS) and 351 patients with stable coronary artery disease,
the prevalence of attenuated plaque was 43.8% and 27.9%, respectively, and its adjusted odds ratio (OR) to predict post-PCI
TIMI<3 was 5.9 (95% confidence interval [CI]: 2.4 to 14.5) and 6.6 (95% CI: 1.4 to 32.1), respectively.
Kimura S, Kakuta T, Yonetsu T, et al. Clinical significance of echo signal attenuation on intravascular ultrasound in patients with coronary artery disease. Circ
Cardiovasc Interv. 2009;2(5):444-454. doi:10.1161/CIRCINTERVENTIONS.108.821124](https://image.slidesharecdn.com/ivusvsoct-210220122303/85/IVUS-v-s-OCT-for-Coronary-Revascularization-16-320.jpg)