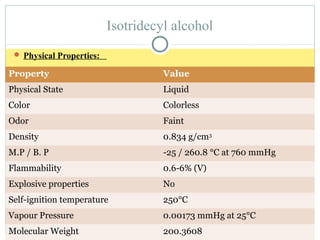
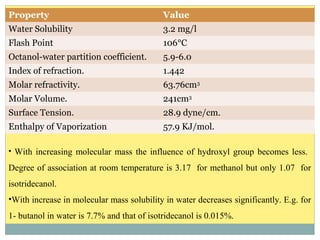
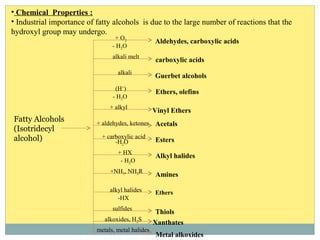
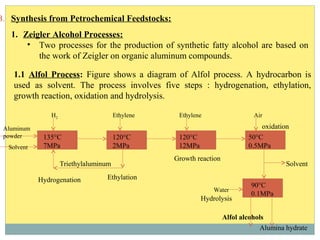
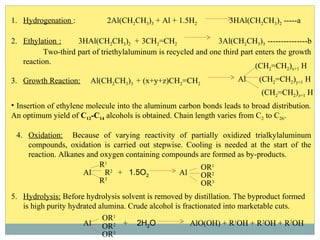
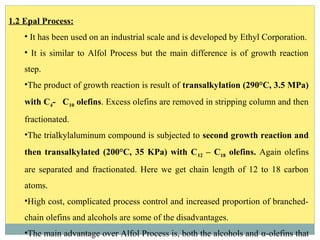
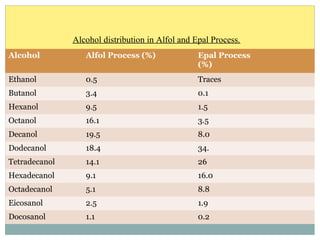
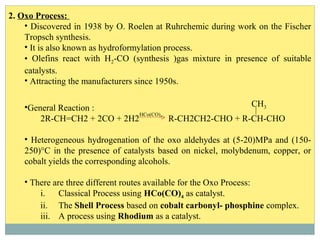
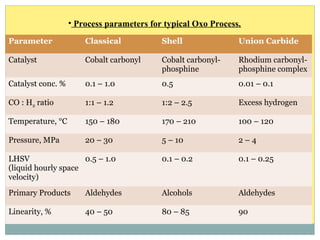
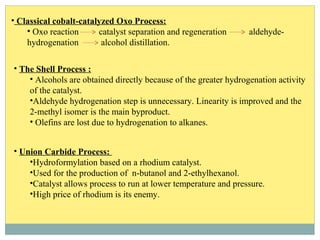
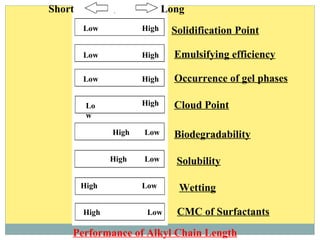
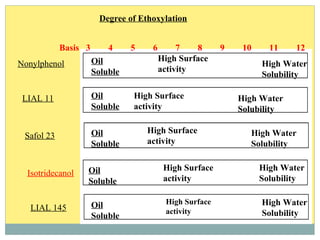
Isotridecyl alcohol is a fatty alcohol with the molecular formula C13H28O. It is classified as a fatty alcohol due to its carbon chain length between C6-C22. Fatty alcohols can be produced through natural sources like plant and animal fats or synthetically from petrochemical feedstocks. The Ziegler and Oxo processes are two common synthetic routes, producing either straight or branched chain alcohols. Isotridecyl alcohol is used to produce surfactants and has applications in detergents, cleaners, and personal care products due to its ability to reduce surface tension.