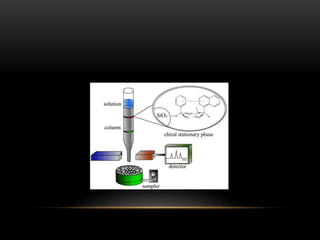
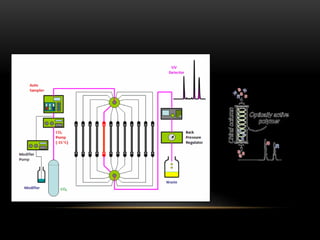
Flash chromatography is a medium-pressure liquid chromatography technique that uses slightly smaller silica gel particles and pressurized gas to drive solvents through columns more rapidly than gravity-fed chromatography. It allows for efficient separation and purification of compounds. Chiral chromatography separates chiral substances like enantiomers using chiral stationary phases or mobile phase additives. These techniques form diastereomers that can be separated based on differences in their physical properties. Flash and chiral chromatography are useful for natural product purification, organic synthesis, and pharmaceutical applications.