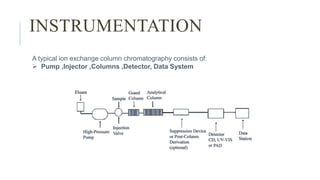
The document provides an overview of ion-exchange chromatography, explaining its principles, instrumentation, applications, advantages, and disadvantages. This analytical technique separates charged molecules based on their affinity for ion exchangers, with various components including a high-pressure pump, injector, columns, detectors, and data systems. While it is effective for purifying and analyzing a wide range of charged compounds, challenges include buffer requirements and variability in column performance.