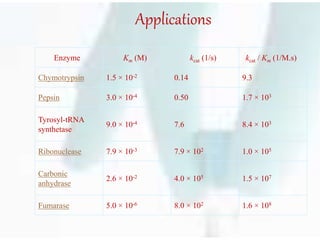
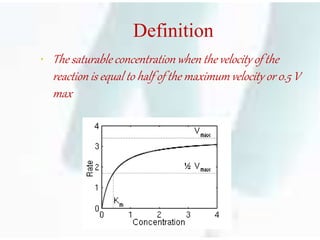
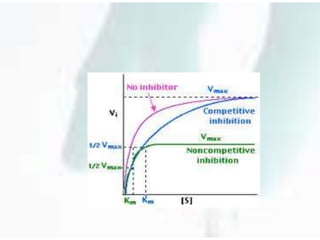
The document discusses the Michaelis-Menten kinetic characteristics, emphasizing the nonlinear pharmacokinetics of drugs that depend on enzyme saturation and transport systems. It outlines various factors contributing to non-linearity in drug absorption, distribution, metabolism, and excretion, while providing equations and assumptions related to enzyme activity. Additionally, it presents applications of Michaelis-Menten kinetics in different fields outside of biochemistry.













![Equilibrium approximation
• In their original analysis, Michaelis and Menten assumed
that the substrate is in instantaneous chemical
equilibrium with the complex, and thus kf[E][S]
= kr[ES]. Combining this relationship with the enzyme
conservation law, the concentration of complex is](https://image.slidesharecdn.com/michaelismentenkinetics-200615042349/85/Michaelis-menten-kinetics-Nonlinear-Pharmacokinetics-18-320.jpg)
![Contd…
• where Kd = kr / kf is the dissociation
constant for the enzyme-substrate complex.
Hence the velocity v of the reaction – the
rate at which P is formed – is
• where Vmax = kcat[E]0 is the maximum
reaction velocity.](https://image.slidesharecdn.com/michaelismentenkinetics-200615042349/85/Michaelis-menten-kinetics-Nonlinear-Pharmacokinetics-19-320.jpg)

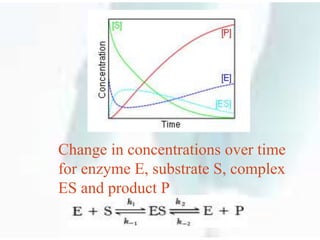
![In this mechanism, the enzyme E is
a catalyst, which only facilitates the
reaction, so its total concentration, free
plus combined, [E] + [ES] = [E]0 is a
constant. This conservation law can also
be obtained by adding the second and
third equations above](https://image.slidesharecdn.com/michaelismentenkinetics-200615042349/85/Michaelis-menten-kinetics-Nonlinear-Pharmacokinetics-21-320.jpg)