Corrosion and Environmental Degradation of Materials-3.pdf
- 1. 1 Dr. P. Justin, M.Sc, M.Tech, Ph.D
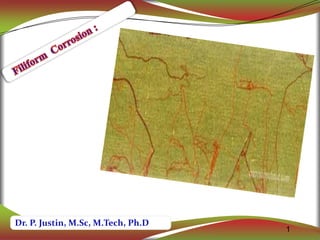
- 2. 2 2 Filiform corrosion is a type of localized corrosion that occurs under some thin coatings in the form of randomly distributed thread like filaments. Filiform corrosion is also known as “underfilm corrosion” or “filamentary corrosion”.
- 3. 11/7/2018 Filiform corrosion preimarily between 65 and 90% relative humidity. If relative humidity is lower than 65%,the metal is unaffected;at more than 90% humidity orrosion primarily appears as blistering.
- 4. 4 Filiform corrosion has been observed on surfaces of coated steel , magnesium , and aluminum of with thin coatings of tin , silver ,gold ,phosphate , enamel and lacquer. Filiform form has been observed on paper-backed aluminum foils Attak of enameled or laquered surfaces of food and beverage ans that have been exposed to the atmosphere.
- 5. The mechanism has a number of characteristics that are similar to crevice corrosion. The coating allows oxygen and water to migrate through it . The concentration of dissolved oxygen becomes highest at the back of the head and this region becomes the cathode. Oxygen becomes depleted at the head . This region becomes the anode. Metal ion formation and dissolution proceeds at the head while oxygen is reduced closer to the tail.
- 6. 6 Proper surface retreatment is required . Use brittle coatings . Control the relative humidity. Very low water permeability hold some promise in preventing filiform corrosion
- 7. 11/7/2018
- 8. 1
- 9. 2 Galvanic corrosion occurs when two dissimilar metals (ex. Zinc and copper ) are electrically connected and exposed to an electrolyte, the metal higher in electrochemical series undergoes corrosion. In this process the more active metal (with more negative electrode potential) act as a anode. Less active metal (with less negative electrode potential) act as a cathode.
- 10. 3 Galvanic corrosion of aluminum shielding in buried telephone cables. Corrosion of steel pipe with brass fittings. Corrosion of the body of the ship in contact with brass or bronze propellers.
- 11. 4 corrosion between tubes and tube sheet in heat exchangers. Galvanic corrosion of steel coated with copper due to the defects in copper coating. Galvanic corrosion of the statue of liberty. Galvanic corrosion inside horizontal stabilizers in aircrafts.
- 12. 5
- 13. 6 For the formation of galvanic cell, the following components are required: (1) A cathode (2) An anode (3)An electrolyte (4)A metallic path for the electron current.
- 14. 7
- 15. Moisture acts as an electrolyte and the metal surface provides a metallic path for the electron current to travel. A piece of copper is the cathode. Galvanic cell will formed and galvanic corrosion proceeds. In the case of copper and steel pipe joint, copper has a more positive potential according to the emf series, hence, it acts as a cathode. Where as Iron has a negative potential in the emf series (—0.440 V), hence, it is the anode
- 16. 9 The following factors significantly affect the magnitude of galvanic corrosion : a)Position of metals in the galvanic series. b) The nature of the environment. c)Area , distance and geometric effects. For galvanic corrosion the magnitude of galvanic corrosion primarily depends on how much potential difference exists between two metals. For a particular environment, the metals selected should be close to each other in galvanic series to minimize galvanic corrosion . Active metals should not be joined with passive metals. Al should not be joined to Fe as Al being more active would tend to corrode.
- 17. 10 Galvanic corrosion of buried material is reduced because of the increased resistivity of soil. Tantalum is very corrosion resistant metal. It is anodic platinum and carbon, but the cell is active only at the high temperatures. Zn is anode with respect to Fe at room temperature with presence of water, but if the water temperature became higher(60 to 75 C deg.)the polarity will reverse and Fe will be anode(corroded). The environment that surrounds the metal must be consider, water containing copper ions like sea water is likely to form galvanic cell on a steel surface of the tank.
- 18. 11 The anode to cathode area ratio is extremely important as the magnitude of galvanic corrosion is seriously affected by it. For a given amount of current, the metal with the smallest area has the largest current density and, hence, is more damaged if corrosion occurs at it. Similarly, the current density at a large metal is very small. The rate of corrosion increases with the ratio of cathodic to anodic areas. An unfavourable area ratio consists of a large cathode and a small anode. Corrosion of anodic area may be 100 times greater than if the anodic and cathodic areas were equal in size .
- 19. 12 Take the example of steel plates joined by aluminum bolts . Aluminum has a smaller anodic area and steel, has a larger cathodic area. Aluminum is more active in the galvanic series than steel. The current density on aluminum is, therefore, extremely large and serious galvanic corrosion of aluminum takes place .
- 20. 13 It is a known principle that the solution conductivity varies inversely with the length of the conduction path. Most corrosion damage is caused by current which covers short paths.. The greatest galvanic damage is likely to be encountered near the junction of the two metals and severity would be decreased with increased length. If two different metals and the severity would be decreased with increased length. If two different metals are far away from each other, there would be no risk of galvanic corrosion, because of very little current flow.
- 21. 14 Galvanic attack would be restricted for two dissimilar metals in contact with soil of high pH and low in carbon dioxide.
- 22. 15 Geometry of components and their design also influence galvanic corrosion.. As current does not flow around the corners, the geometry of the circuit affects the degree of galvanic corrosion. Any obstacle to polarization would accelerate galvanic corrosion. Component design is also a factor in galvanic corrosion as the current circuit geometry affect the magnitude of galvanic corrosion and the polarization process.
- 23. 16 The nature of non-metallic conductors must be known before their application. Graphite packing around a steel pump shaft can cause galvanic corrosion of the steel shaft if it is wet Many non-metallic materials are cathodic to metals and alloys. For example, impervious graphite used in heat exchanger applications is noble to more active metal.
- 24. 17 Zinc corrodes eventually and it protects the steel substrate both by its barrier effect and also by providing electrons (Zn -> Zn2+ + 2e) into the steel which prevent Fe++ ions from escaping from the steel (cathodic protection ) Nobel coatings act as a barrier only between the metal substrate and the environment . Nickel, silver, copper, lead ,and chromium are called noble metal coatings. Formation of pores and damage to the noble coating can cause galvanic corrosion of the substrate , as there is no sacrificial cathodic protection of the substrate. Two types of metallic coatings are generally used they are noble and sacrificial type . Zinc coating is an example of sacrificial type.
- 25. 18
- 26. 19 As mentioned above, a positive use of the principle of galvanic corrosion is cathodic protection. In a sacrificial system of cathodic protection, anodes of active metals, like Zn, Mg and Al, are used for protection of steel structures. The sacrificial galvanic anodes provide protection to the less active metals, like steel because they corrode and release electrons. The electrons which are released by the corroding metals enter the steel structures, which become cathodic and, therefore, do not corrode . This system of cathodic protection is based on galvanic corrosion, however, in this case a beneficial use is the result of galvanic corrosion.
- 27. Use inhibitors in aqueous system and eliminate cathodic depolarizers. Select metals as close together as far as possible, in the galvanic series. If dissimilar metals are used, insulate them each other. Apply coatings with judgement. Avoid un favourable effect small anode- large cathode combination.
- 28. 21 Install a third metal that is anodic to both metals in the galvanic contact. In designing use replaceable parts so that only the corroded parts could be replaced instead of the whole assembly Avoid threaded joints for materials.
- 30. 1
- 31. 2 Localized attack adjacent to grain boundaries with relatively little corrosion of grains Metals are usually “polycrystalline” . . . an assemblage of single-crystal grains separated by grain boundaries. The atoms in the grain boundaries are in a distorted lattice (i.e. .Disordered) The higher energies of grain boundary atoms make them slightly more reactive than grains. The grain boundary material, which is a limited area, acts as an anode, and the larger area of grains acts as cathodes. This results in the flow of energy from the small anode area to the large cathode area, which causes rapid attack penetrating deeply into the metal.
- 32. 3 Examples of intergranular corrosion: ➢ Intermetallic compound such as Mg5 Al8,formation at the grain boundaries from a galvanic cell with a alloy matrix and in chloride environment severe intergranular type of corrosion occur. ➢ Intergranular corrosion can occur in many alloys systems such as stainless steel , nickel base and aluminum base alloys.
- 33. 4 • Impurities at the grain boundaries. • Enrichment of one of the alloying element. • Depletion of one of the elements that affects its corrosion resistance in grain boundary areas. • Depletion of chromium in the grain boundary region results in intergranular corrosion. • Small amounts of iron in aluminum, where solubility of iron is low, have been shown to segregate in the grain boundaries and cause intergranular corrosion. • Intergranular corrosion of an alloy may be caused by • The potential difference between the grain boundary regions and any precipitate, intermetallic phases, or impurities that form at the grain boundaries is responsible for higher dissolution rates at these regions.
- 34. 5 • The actual mechanism differ from one alloy system to another. • Some precipitates from preferentially at grain boundaries as a result of production, fabrication and welding at elevated temperature. • Small amounts of iron in aluminum, where solubility of iron is low, have been shown to segregate in the grain boundaries and cause intergranular corrosion.
- 35. 6 • The almost universally accepted theory for IGC is based on impoverishment or depletion of chromium in the grain-boundary areas. • In the temperature range indicated , Cr23C6 (and carbon) is virtually insoluble and precipitates out of solid solution if carbon content is about 0.02% or higher. • The chromium is thereby removed from solid solution , and the result is metal with lowered chromium content in the area adjacent to the grain boundaries. • The chromium-depleted zone near the grain boundary is corroded because it does not contain sufficient corrosion resistance to resist attack in many corrosive measurements. • The net effect is rapid attack in the impoverished area , with little or no attack on the grains.
- 36. 7
- 37. 8 ➢ On exposure to a corrosive environment, failure of an austenitic stainless steel weld is called weld decay.
- 38. ➢ Spot welding, in which the metal is rapidly heated by a momentary electric current followed by a naturally rapid cooling, does not cause sensitization. Arc welding can cause damage with the effect being greater. ➢ Sensitizing temperatures are reached some millimeters away from the weld metal itself, with the latter being at the melting point or above. ➢ The metal will be in a sensitizing zone for a longer time if gas welding is used, hence a greater amount of carbide would precipitate .Thus steel will be attacked by intergranular corrosion. ➢ If a metal is weld at the particular area near to melting point. At the place a temperature gradient is act and the carbon is diffuse and form chromium carbide precipitates. ➢ Cr atoms come to grain boundaries and form Cr carbides. Because the formation of Cr23c6 is more stable than Fe-C precipitates. Cr carbides are hard and act as crack initiations.
- 39. 10 ➢ Use low carbon content grade stainless steels Eg.,316 L , 304 L – 0.3% wt ,So carbide formation is minimal. ➢ Use a stabilized grade of SS , which contain carbide forming elements such as Nb or Ti and Titanium , which form titanium carbide , Niobium Carbide and tantalum carbide preferentially to chromium carbide. ➢ Heat treatment to re-dissolve the carbides (post welding heat treatment) ➢ Weak corrosive conditions do not cause IGC ➢ Low acidity (high pH) will generally reduce the susceptibility to IGC.
- 40. ➢ Stabilized austenitic stainless steels may become susceptible to a localized form of intergranular corrosion known as knife-line attack or knife-line corrosion. ➢ During welding, the base metal immediately adjacent to the fusion line is heated to temperatures high enough to dissolve the stabilizing carbides, but the cooling rate is rapid enough to prevent carbide precipitation. ➢ Subsequent welding passes reheat his narrow area into the temperature range in which both the stabilizing carbide and the chromium carbide can precipitate. ➢ The precipitation of chromium carbide leaves the narrow band adjacent to the fusion line susceptible to intergranular corrosion. ➢ Knife-line attack can be avoided by the proper choice of welding variables and by the use of stabilizing heat treatments.
- 41. 12 Precipitation reactions in types 304 and 347 steels ➢ On exposure to a corrosive environment, failure stainless steel weld is called weld decay.
- 42. 13 ➢ Knife line attack can be avoided ➢ By the proper choice of welding variable. ➢ By the proper choice of welding materials. ➢ By the use of stabilizing heat treatment.
- 43. 14 Factors Effect on intergranular corrosion time as time increases in the sensation effect of IGC will increase. temperature as temperature increases effect of IGC will decrease. Element Intergranular corrosion C Increases Cr decreases Mo decreases Ni decreases N Depends on content of steel. Nitrogen must be considered when titanium used as stabilizer, not because the precipitation of chromium nitride is a problem in austenitic steels, but because titanium nitride is very stable.
- 44. 15 1. Intergranular corrosion is a) restricted to stainless steels b) not restricted to stainless steel but includes nickel-base alloys also c) restricted to alloys containing low carbon content 2. Which of the two stainless steels, ferritic or austenitic, would sensitize more under the given conditions? a) Ferritic steel when quenched from 800° C or higher b) Ferritic steels on slow cooling from 800°C c) Austenitic steels when slowly cooled through 500°C 3. Sensitization in ferritic stainless steels can be eliminated by a) lowering the carbon content to 50 ppm b) lowering chromium to less than 12% c) lowering the amount of interstitial carbon and nitrogen to 50 ppm and 150 ppm, respectively
- 45. 16
- 46. 1
- 47. 2 Selective leaching is a corrosion type in some solid solutions alloys,when in suitable conditions one or more components of the alloys is preferentially leached from the material. Common dealloying examples are: Decarburization,Dezincification ,Denickelification, graphitic corrosion Decobalification. Alloys are exposed to corrosive experience selective leaching and the more active constituent results in the loss of structural stability and mechanical strength. The most susceptible alloys are the ones containing metals and high distance between each other in the galvanic series, examples: CU and Zinc in brass. The elements most typically undergoing selective removal are Zinc , Aluminium , iron , cobalt, chromium and others. Selective leaching is also called dealloying, parting selective corrosion and demetalification
- 48. 3 Causes of dealloying: ➢Different metals and alloys have different electrochemical potentials (or corrosion potentials) in the same electrolyte. ➢ Modern alloys contain a number of different alloying elements that exhibit different corrosion potentials. ➢ The potential difference between the alloying elements is the driving force for the preferential attack on more “active “ elements in the alloy. ➢ e.g. In the case of dezincification of brass (Cu & Zn), Zinc is preferentially leached out of the copper -Zinc alloy, leaving behind a copper rich surface layer that is porous and brittle
- 49. 4 Dezincification is the selective leaching of zinc from zinc containing alloys like brasses. It mainly occurs in alloys containing less than 85% copper after extended service in water containing dissolved oxygen, sulphur and carbondioxide. ➢ Impurities segregation at the grain boundaries. Dezincification generally takes place in water under stagnant conditions. copper-zinc alloys containing more than 15% zinc are susceptible to dezincification. Dezincification can be observed by naked eyes, because the alloy changes in color from yellow to red. Two types of dezincification are commonly observed : 1.Uniform type(layer type) 2.Localized or plug type. ➢ Impurities segregation at the grain boundaries.
- 50. 5 (1) Uniform layer type dezincification occurs in tooth of gear wheel. It also occurs in the inner surface of brass heat exchangers tubes when exposed to water at pH=8.0 and temperature range 31-49˚c. Brass with high Zinc content in acidic environment is highly prone to uniform dezincification. In this type corrosion result in relatively uniform zone of dezincified material , with the underlaying material remains unaffected. (2) Plug type is found particularly in α-brass heat exchangers pipes . If the heat exchangers is not cleaned and dried, differential aeration cells are formed in which the brass dissolves. The corroded region is filled with the reprecipitated copper.
- 51. 6 There are three steps of mechanism for dezincification 1. First entire brass alloy is dissolved. 2. The noble metal is replated (e.g. cu in brass) 1. Addition of passivating inhibitors: 3. Active metal is leached away. (e.g. Zn in brass)
- 52. 7 Zinc is quite reactive to corrosive environment while copper is more noble metal. The analysis of dezincified area shows 90-95% copper with present as copper oxide. When oxygen is present then it also enters into cathodic reaction and increase rate of attack. Zinc can corrode slowly in corrosive environment and leaching of zinc occurs in brass which makes porous structure of copper. This mechanism is called dezincification.
- 53. 8 The bases of the dissolution and redeposition mechanism
- 54. 9 Reducing the aggressiveness of the environment (i.e. oxygen removal). Use less susceptible material Addition of small amounts of As, Sb and P as inhibitors. Addition of 1% Sn to a 70-30 brass which improve resistance towards dezincification. Cupronickel (70-90% Cu, 30-10%Ni) is used in corrosive environment)
- 55. 10 Graphite corrosion should not be confused with another term graphitization , which is used to describe the formation of graphite in iron or steel, usually from decomposition of iron carbide at elevated temperatures . Graphite corrosion does not takes occurs in nodular or meallable cast iron. Graphitic corrosion is the deterioration of gray cast iron in which the metallic constituents are selectively leached or converted to corrosion products leaving the graphite intact.
- 56. 11 Alloy Environment Element removed brasses Many water,especially under stagnant conditions Zinc (dezincification) Gray iron Soils,many water Iron (graphitic corrosion) Aluminium bronzes HF acid, acids containing cl¯ ions aluminium Copper nickels High heat flux and low water velocity(in refinery condenser tubes) Nickel(de-nickelification) Monels Hydroflouric and other acids Copper in some acids and nickel in others Alloys of au or Pt with Ni, Cu or Ag Nitric. chromic and sulfuric acids Ni, cu or Ag (parting) Medium carbon and high carbon steels Oxidizing atmosphers, hydrogen at high temperatures Carbon(decarburization) Iron-chromium alloys High-temperature oxidizing atmospheres Chromium, which forms a protective film Nickel- molybdenum alloys Oxygen at high temperature molybdenum
- 57. 12 It is selective leaching of nickel from nickel containing alloys. Most commonly observed in Cu-Ni alloys after extended service in fresh water.
- 58. 13 It is the selective loss of carbon from the surface layer of carbon containing alloy due to reaction with one or more chemical substances in a medium that contacts the surface. It is selective leaching of cobalt from cobalt base alloys, such as stellite (Co-Cr alloys), or from cemented carbides.
- 59. 14 ➢Control environment to minimize the selective leaching (e.g. lowering oxygen content). ➢ Select metals/alloys that are more resistant to dealloying. E.g. inhibited brass is more resistant to dezincification that alpha brass, ductile iron is more resistant to graphitic corrosion than gray cast iron. ➢ Use cathodic protection. ➢ Use protective coating to protect surfaces.
- 60. 15