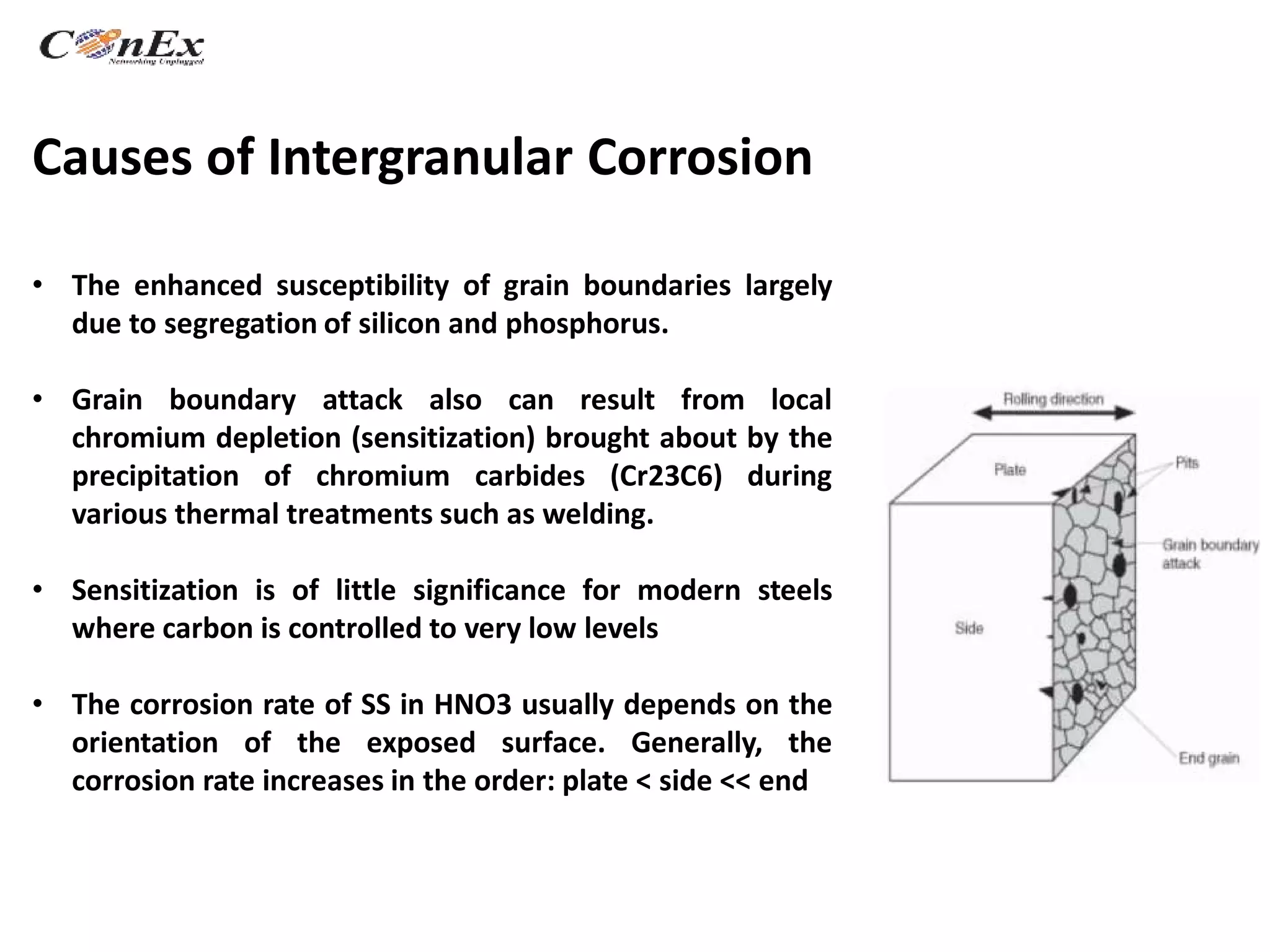
Dr. Shagufta Khan presented on understanding end grain corrosion in austenitic stainless steels. End grain corrosion occurs preferentially at grain boundaries and is caused by segregation of elements like silicon and phosphorus along the boundaries. It is a problem in nuclear reprocessing plants where stainless steel tubing is exposed to highly oxidizing nitric acid. Laser remelting and weld overlays can be used to control end grain corrosion by modifying the microstructure and masking susceptible material, while solution annealing homogenizes the material's structure.

![Dr Shagufta Khan
Present Company: AMCO Integrity Pty Ltd
Name & : Dr Shagufta Khan
Designation: Director Asset Integrity and Corrosion
Academic
Qualification: Ph.D. Corrosion engineering
Specialized Knowledge: Asset Integrity and Corrosion
Achievements: 18 Publications in international journals and conference proceedings
Guest editor for [Sustainability] Special Issue - Sustainable Materials,
Manufacturing and Design.
Key points of the Paper](https://image.slidesharecdn.com/igc-210403024906/75/Intergranular-corrosion-in-SS-tubes-2-2048.jpg)