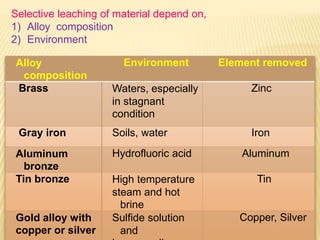
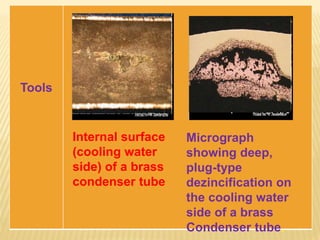
Selective leaching, also called de-alloying or de-metalification, refers to the selective removal of one element from an alloy by corrosion processes. A common example is the dezincification of brass, where zinc is selectively removed leaving a porous copper structure. There are three steps in the mechanism of dezincification: (1) dissolution of the entire alloy, (2) replating of the more noble metal (copper), and (3) leaching away of the active metal (zinc). Dezincification can occur uniformly or in localized plugs and is caused by water containing sulfur, carbon dioxide, and oxygen. Prevention methods include using less susceptible alloys, adding inhibitors like tin