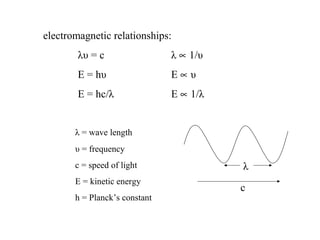
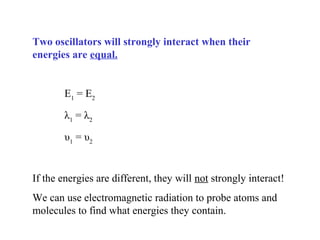
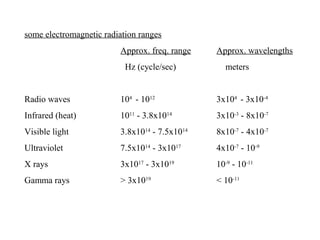
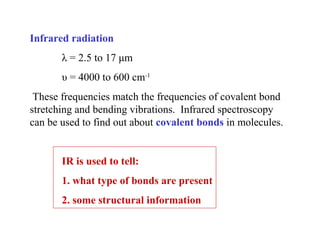
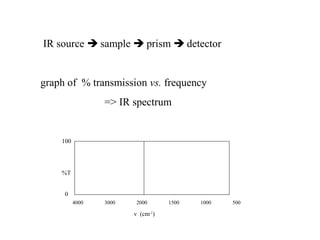
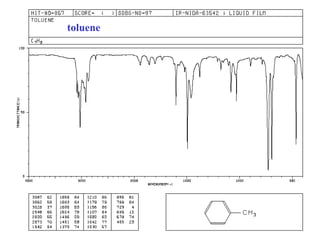
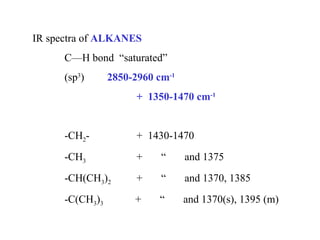
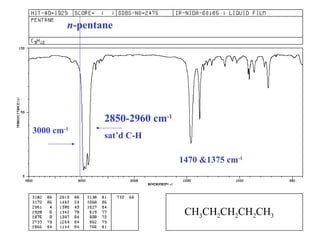
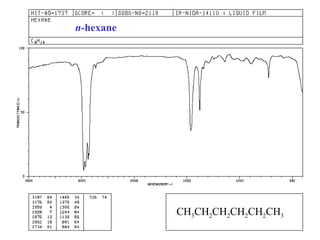
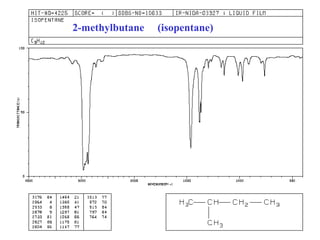
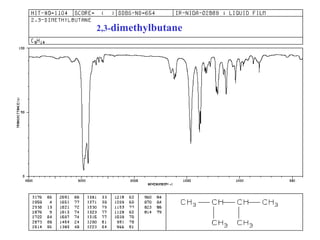
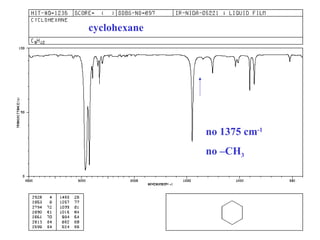
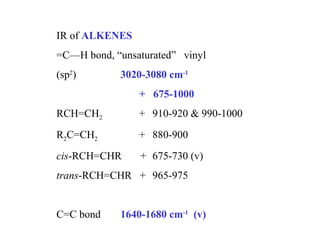
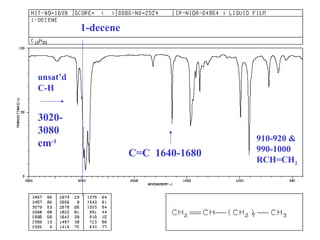
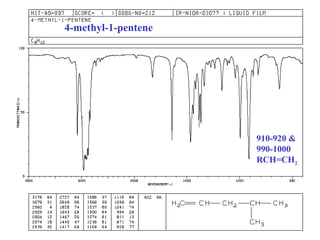
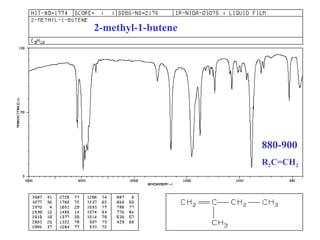
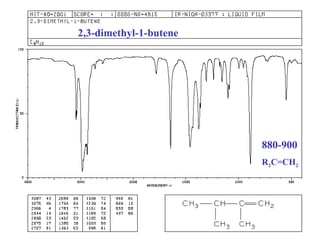
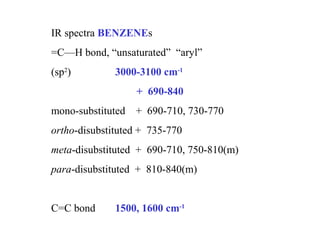
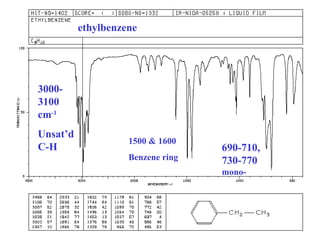
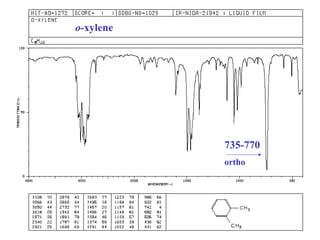
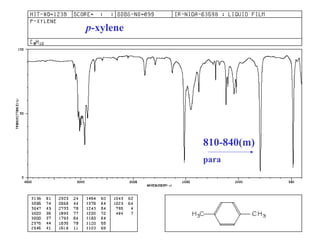
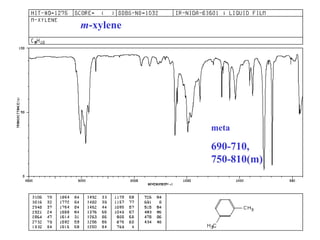
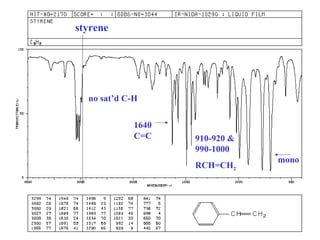
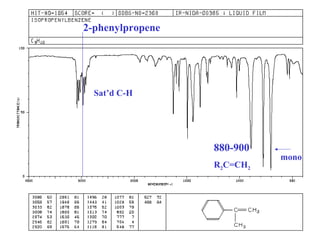
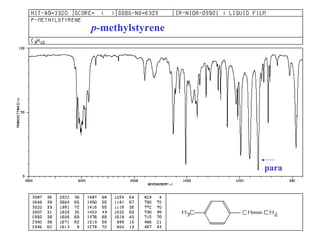
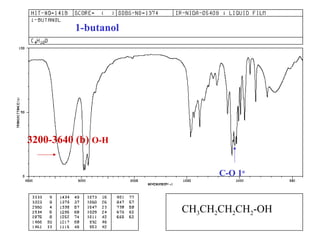
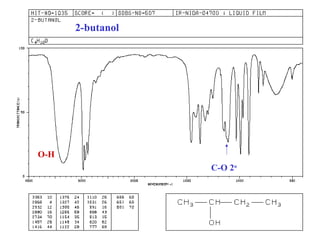
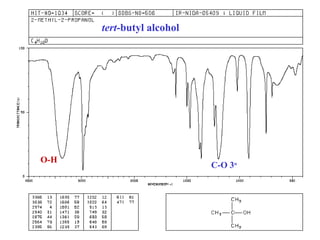
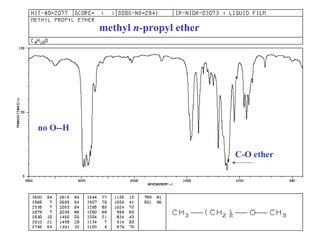
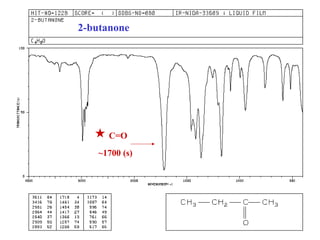
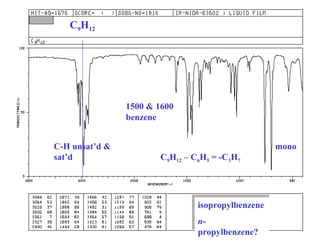
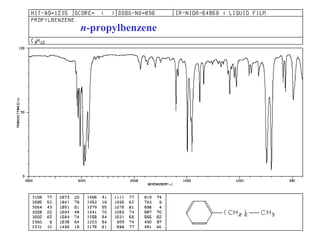
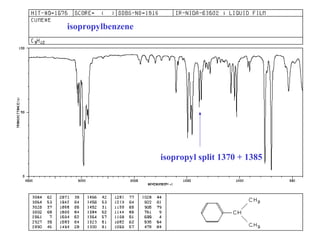
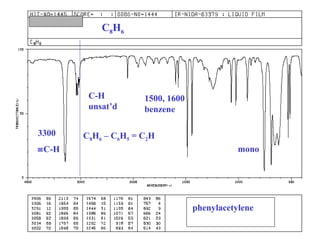
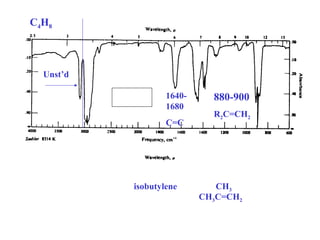
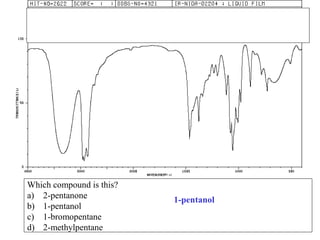
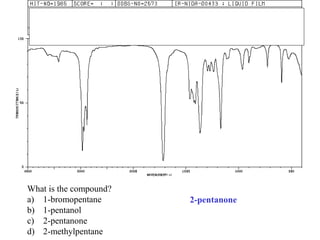
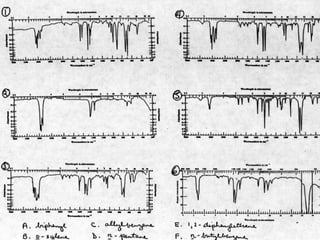
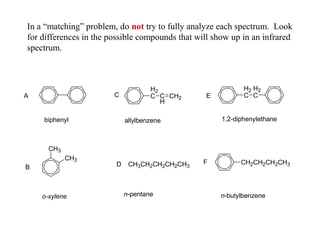
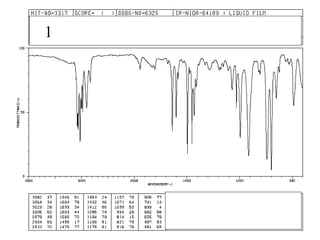
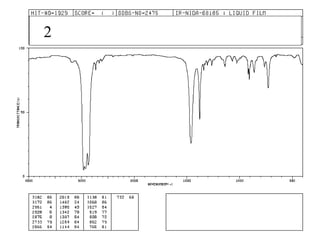
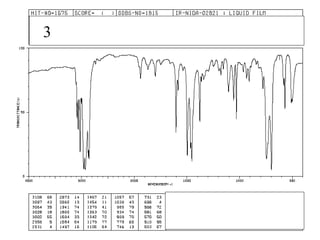
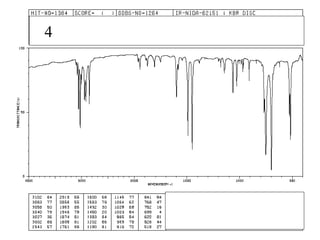
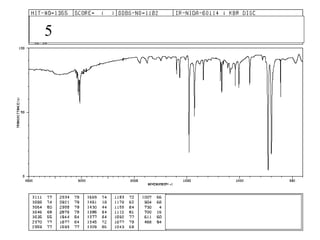
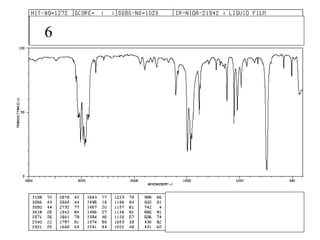
This document provides an overview of infrared spectroscopy and infrared spectra. It discusses how infrared spectroscopy uses electromagnetic radiation to probe molecular vibrations and determine structural information about molecules. Specific molecular vibrations are associated with different functional groups like C-H, C=O, O-H, and C-O bonds. Characteristic absorption frequencies are listed for various bond types in alkanes, alkenes, aromatics, alcohols, ethers and other organic compounds. Example infrared spectra are shown for compounds like n-hexane, cyclohexane, 1-decene, ethylbenzene, and 1-butanol to illustrate absorbances.