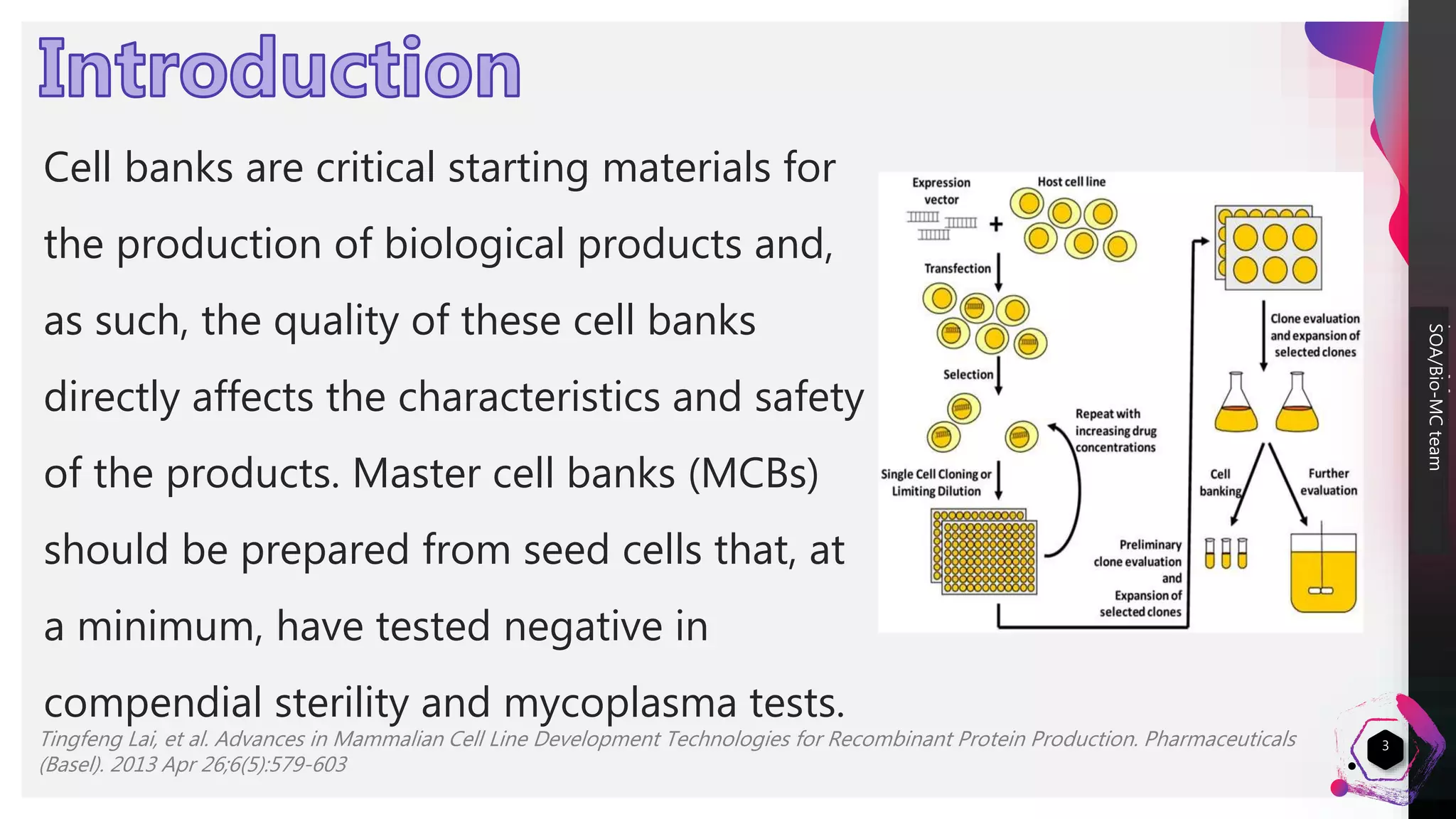
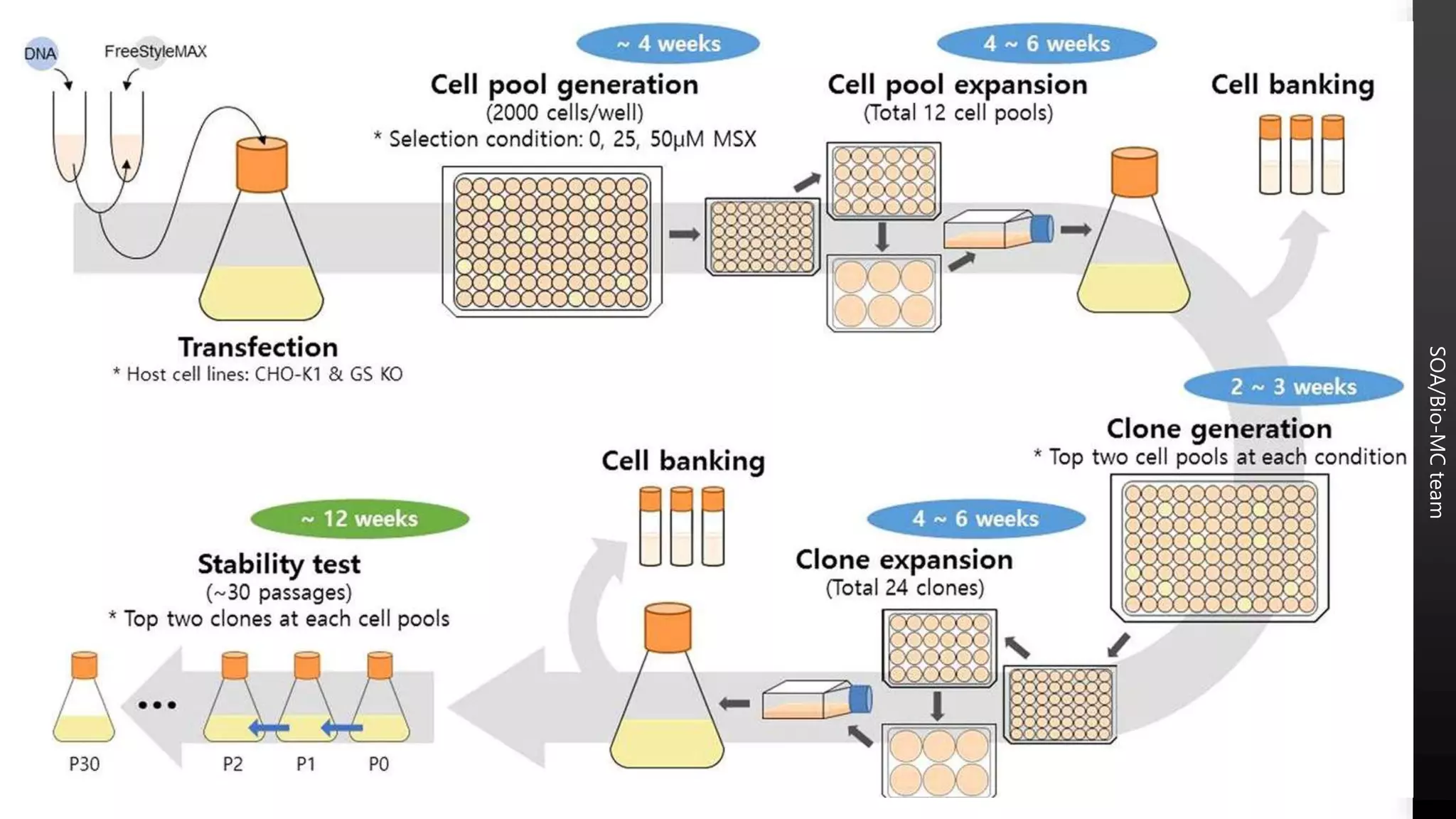
1) Cell lines used for manufacturing biopharmaceuticals must meet strict quality criteria to ensure high quality, stable production. This includes being capable of high productivity, producing molecules with the appropriate structure and quality, and accepting and stably expressing foreign DNA.
2) Master cell banks are critical starting materials that are extensively tested for sterility, mycoplasma, and adventitious agents to ensure safety.
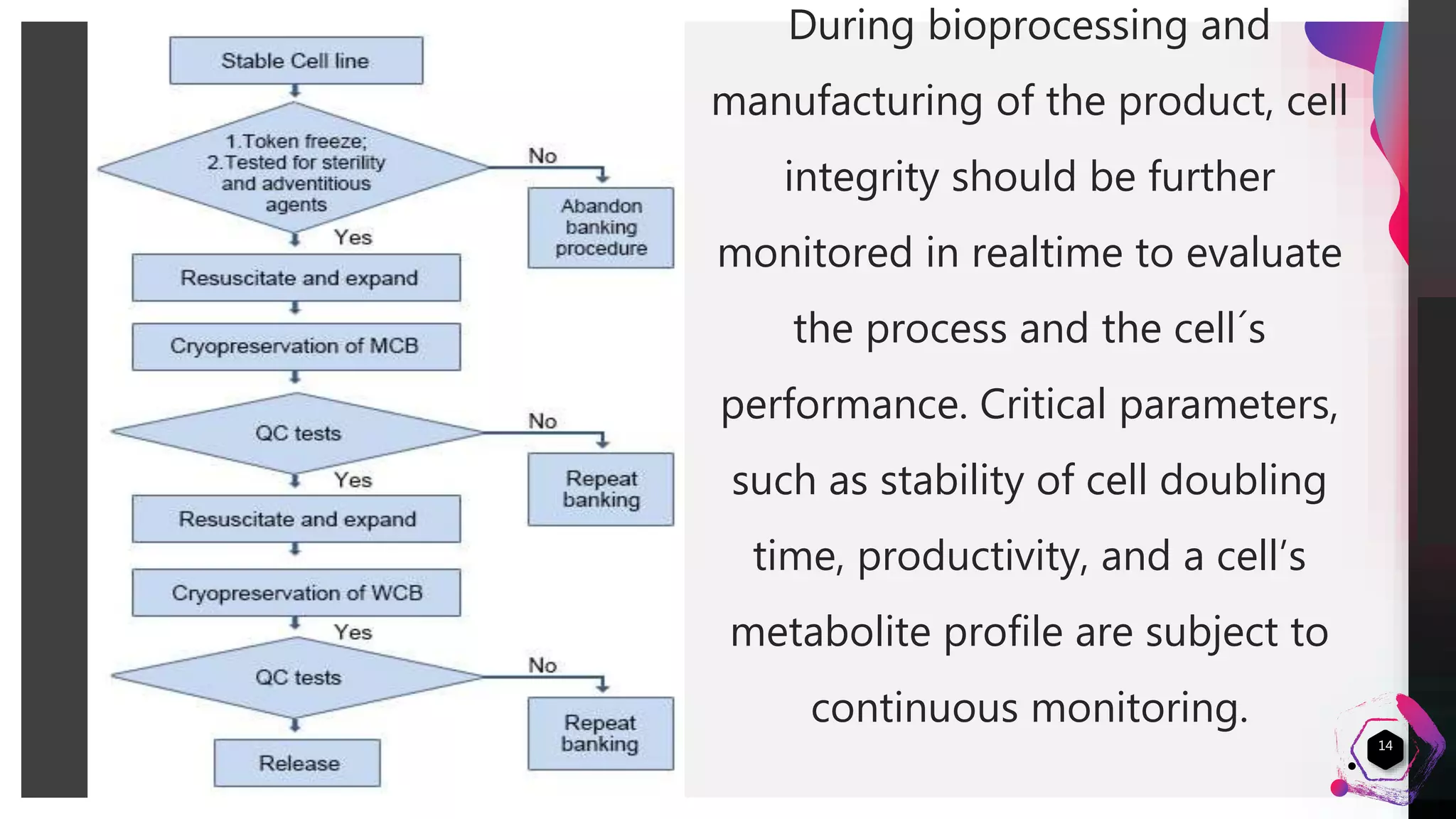
3) Routine monitoring of cell line integrity and performance during biomanufacturing helps evaluate the process and cell line stability over time.