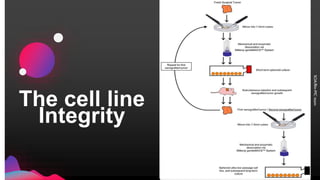
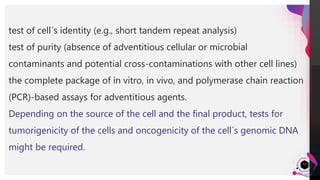
The cell lines used to manufacture biopharmaceuticals must meet strict quality criteria to ensure high-quality, stable products. Master cell banks are critical starting materials, and should be sterile, mycoplasma-free, and tested for identity and purity. Cell lines must stably express the drug with high productivity, appropriate quality and structure, and be robust enough for large-scale production. Comprehensive testing of cell lines and banks is required by guidelines to confirm identity, purity, freedom from contamination, and genetic stability.