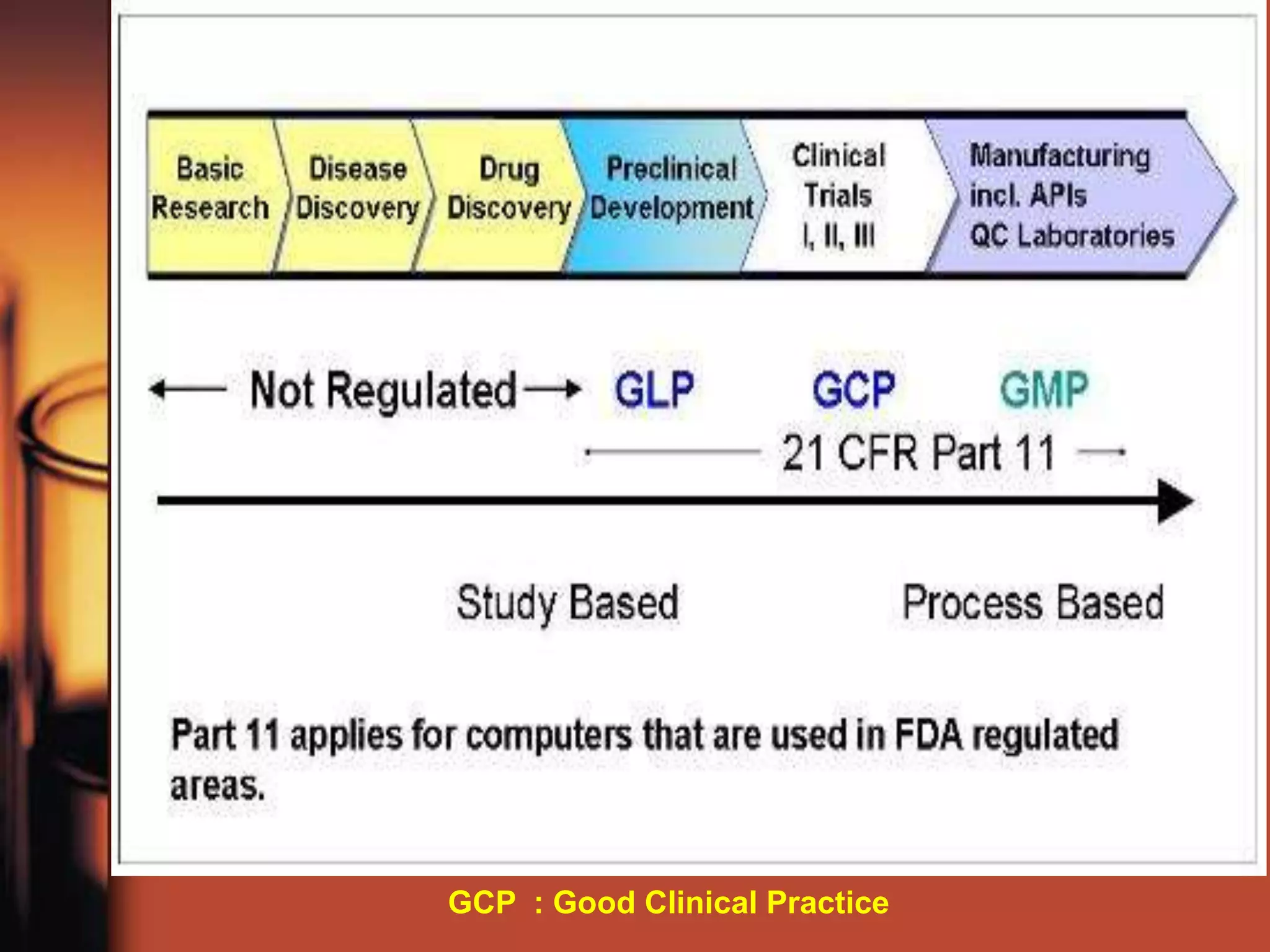
This document outlines Good Laboratory Practice (GLP) standards for non-clinical safety studies. GLP aims to promote quality test data and ensure sound management of laboratory studies, including conduct, reporting, and archiving. GLP applies to non-clinical studies designed to obtain safety data on items to be submitted to regulatory authorities. It covers topics like facilities, equipment, organization, personnel responsibilities, protocols, record keeping, and quality assurance.