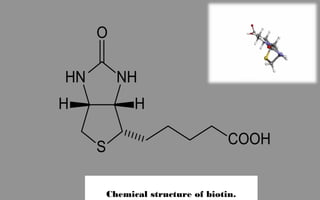
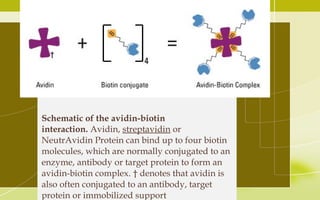
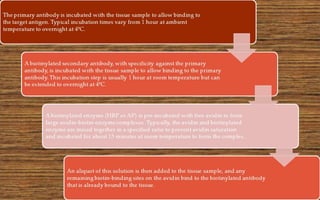
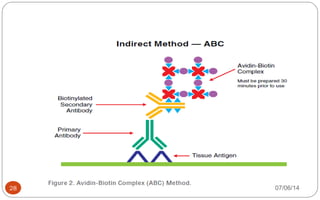
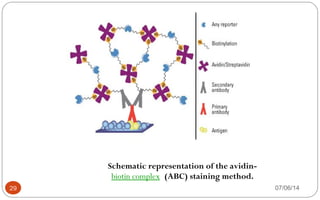
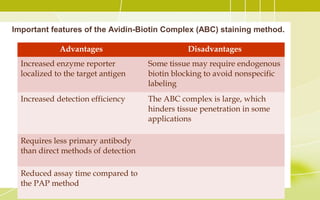
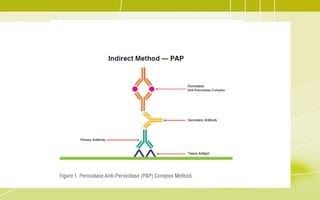
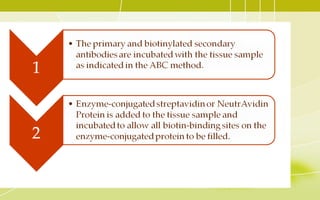
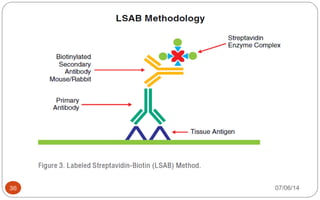
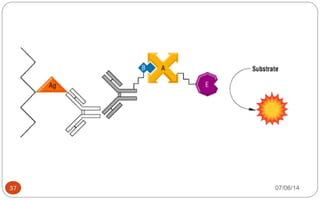
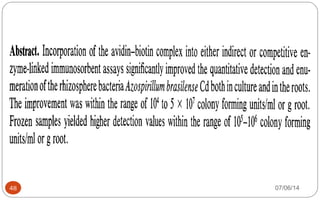
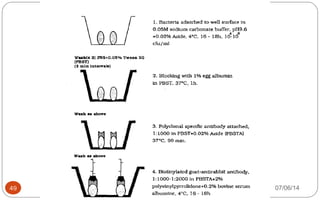
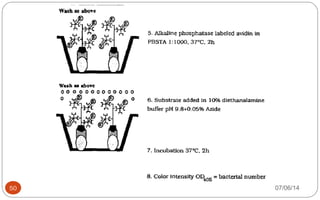
Immunochemistry, specifically immunohistochemistry (IHC), combines histological, immunological, and biochemical techniques to identify specific tissue components through antigen/antibody reactions tagged with visible labels. The avidin-biotin complex is critical in IHC, enabling sensitive assays for detecting various proteins and nucleic acids, while the labeled streptavidin-biotin method enhances detection sensitivity. However, challenges such as nonspecific labeling due to endogenous biotin and the strength of avidin-biotin bonds necessitate careful consideration in assay applications.