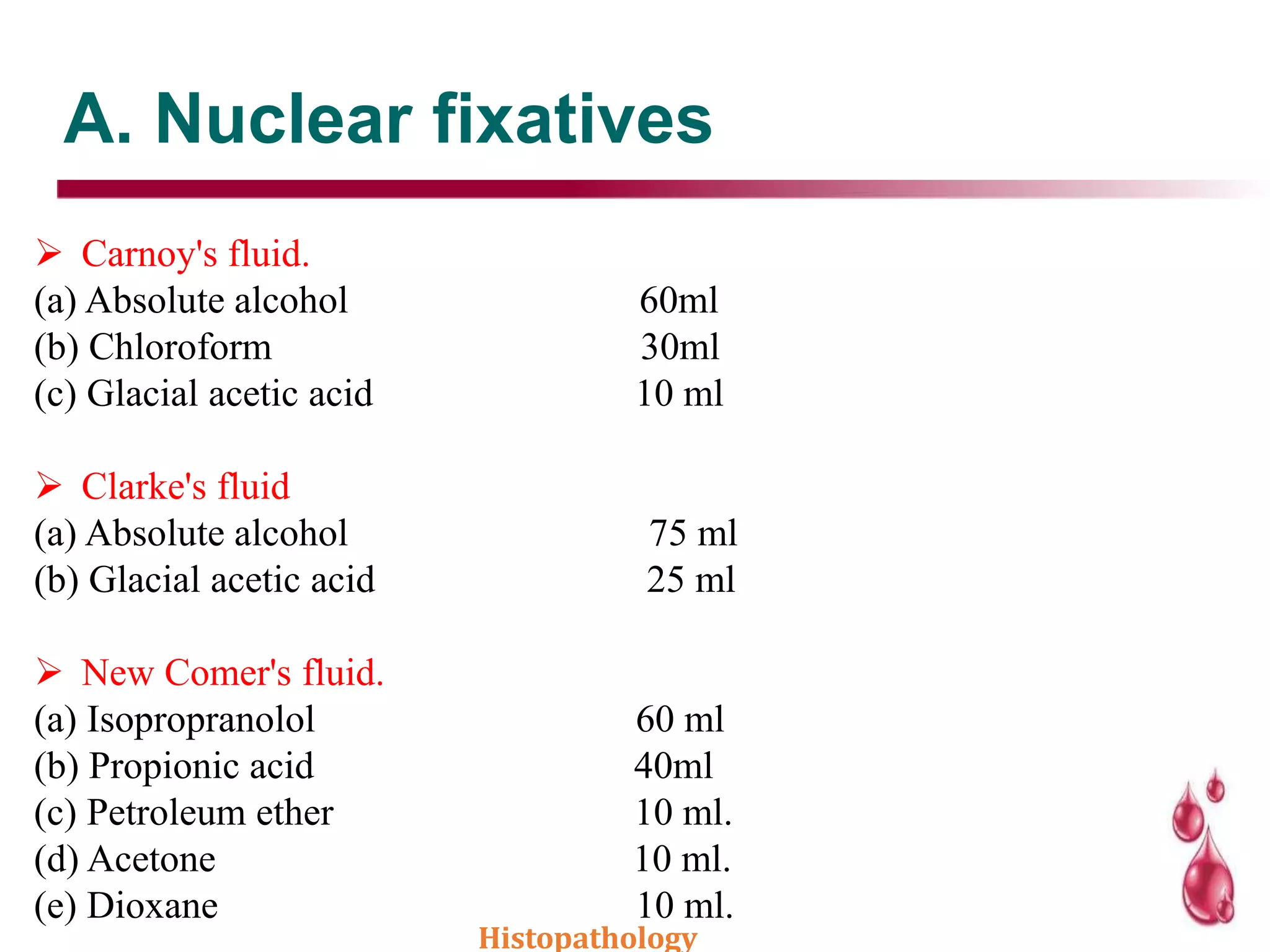
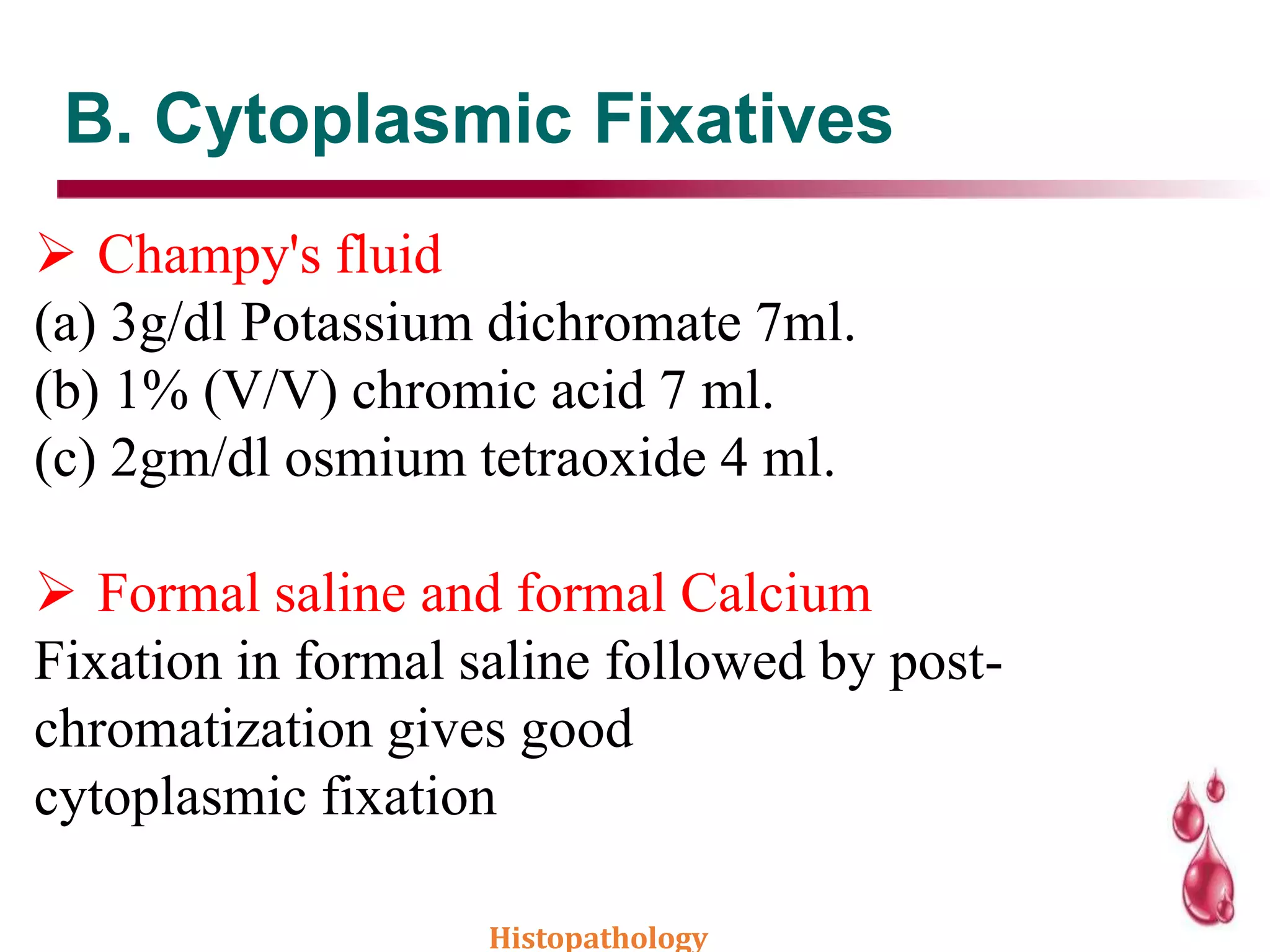
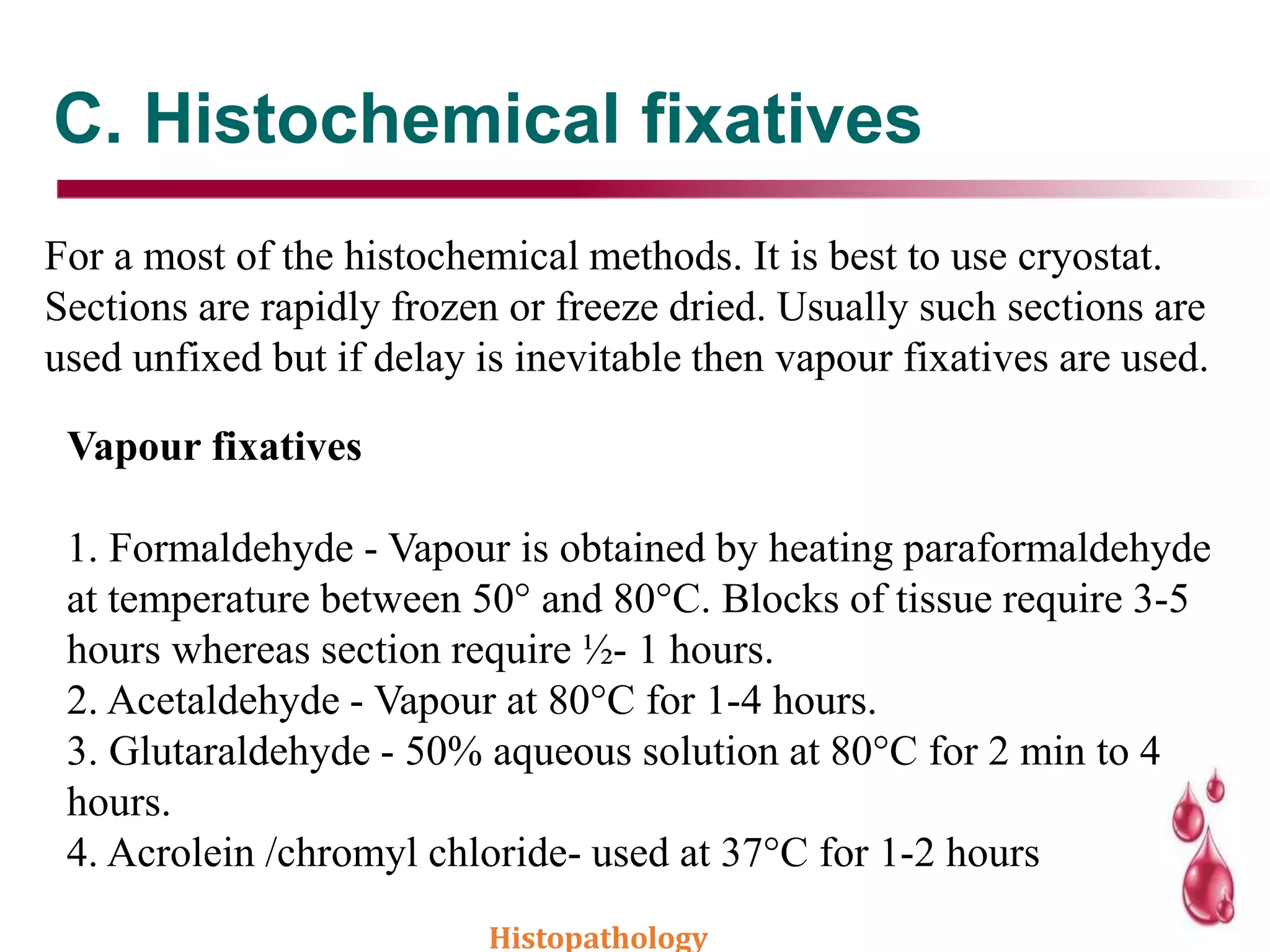
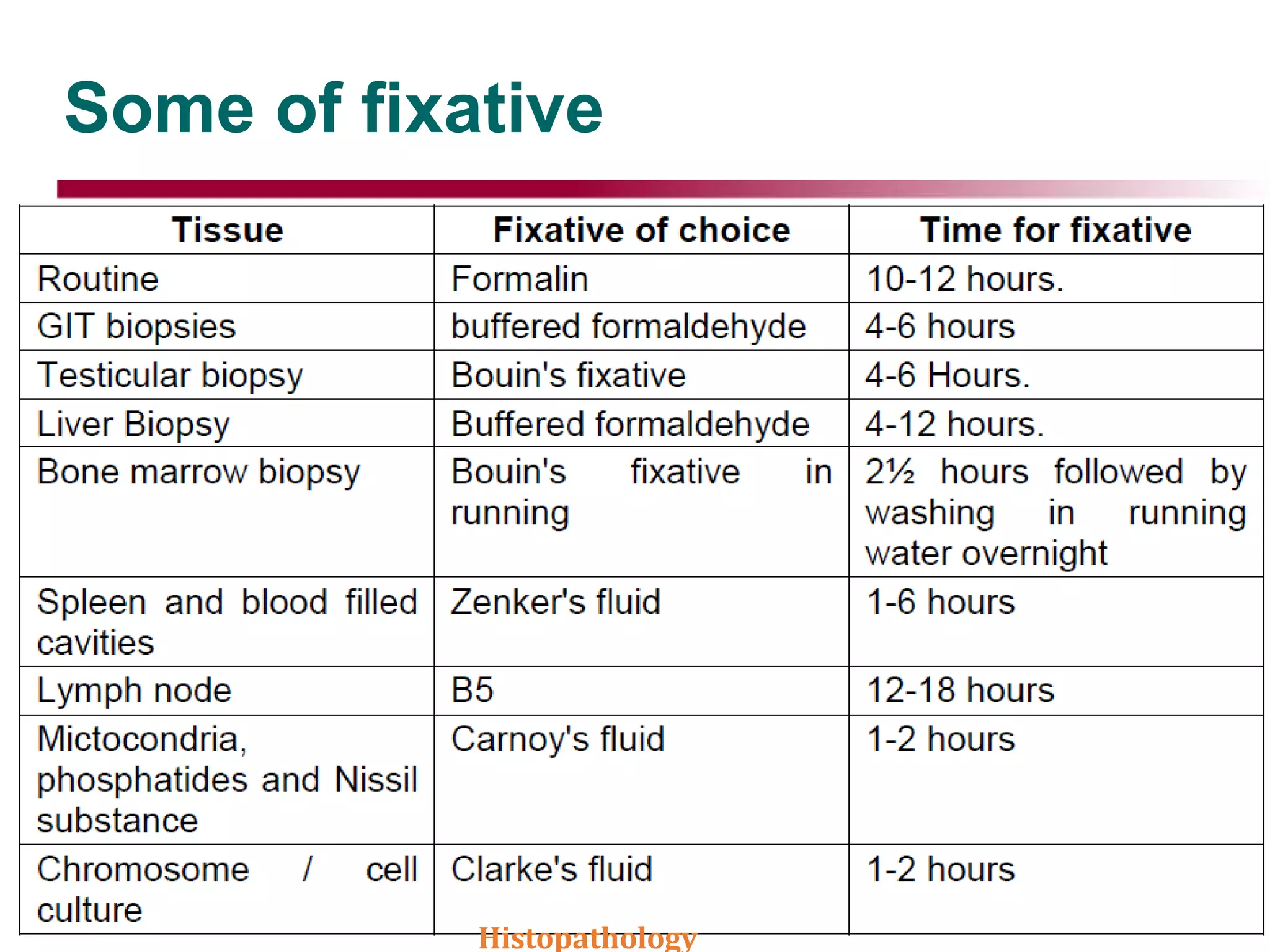
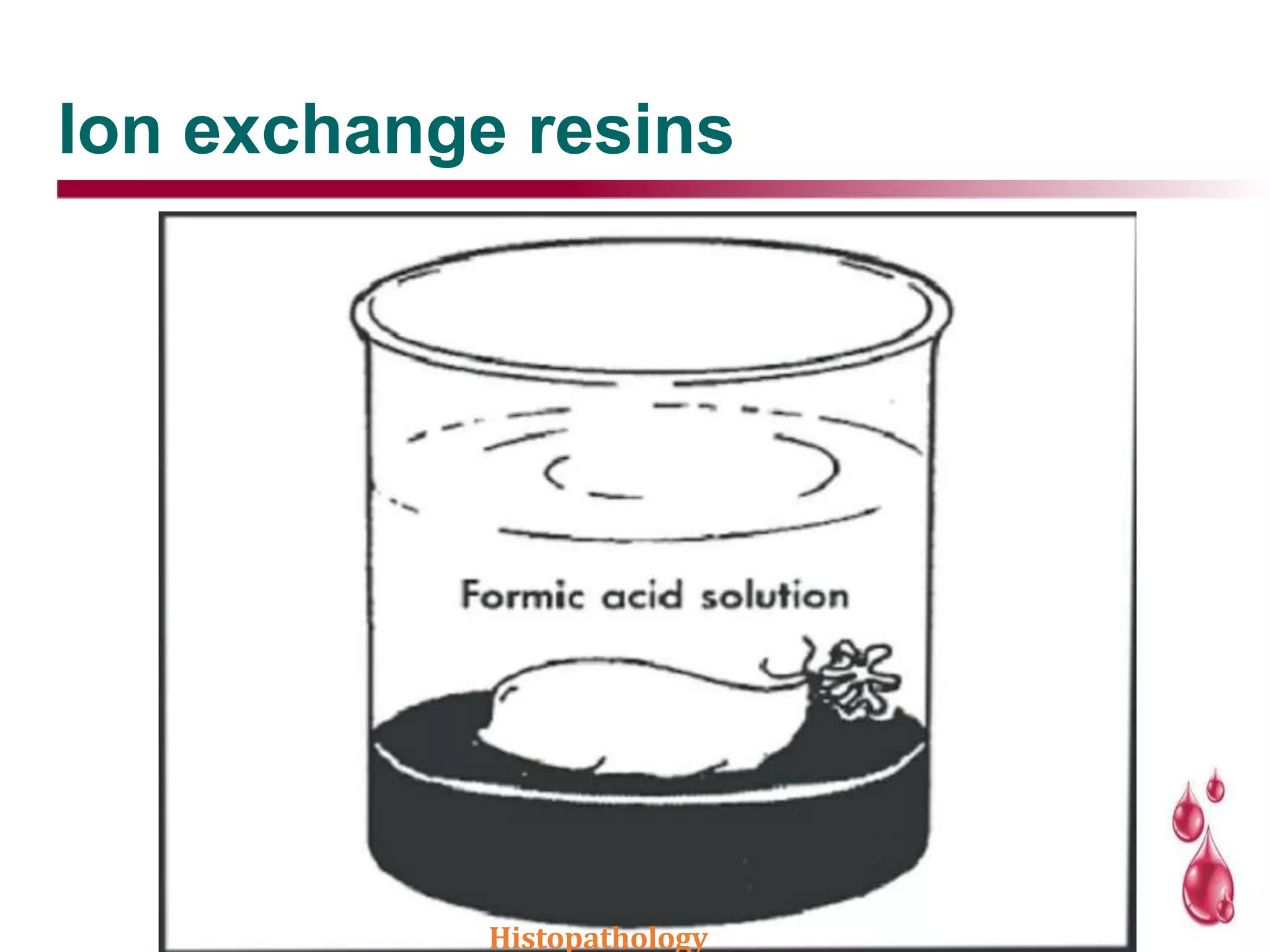
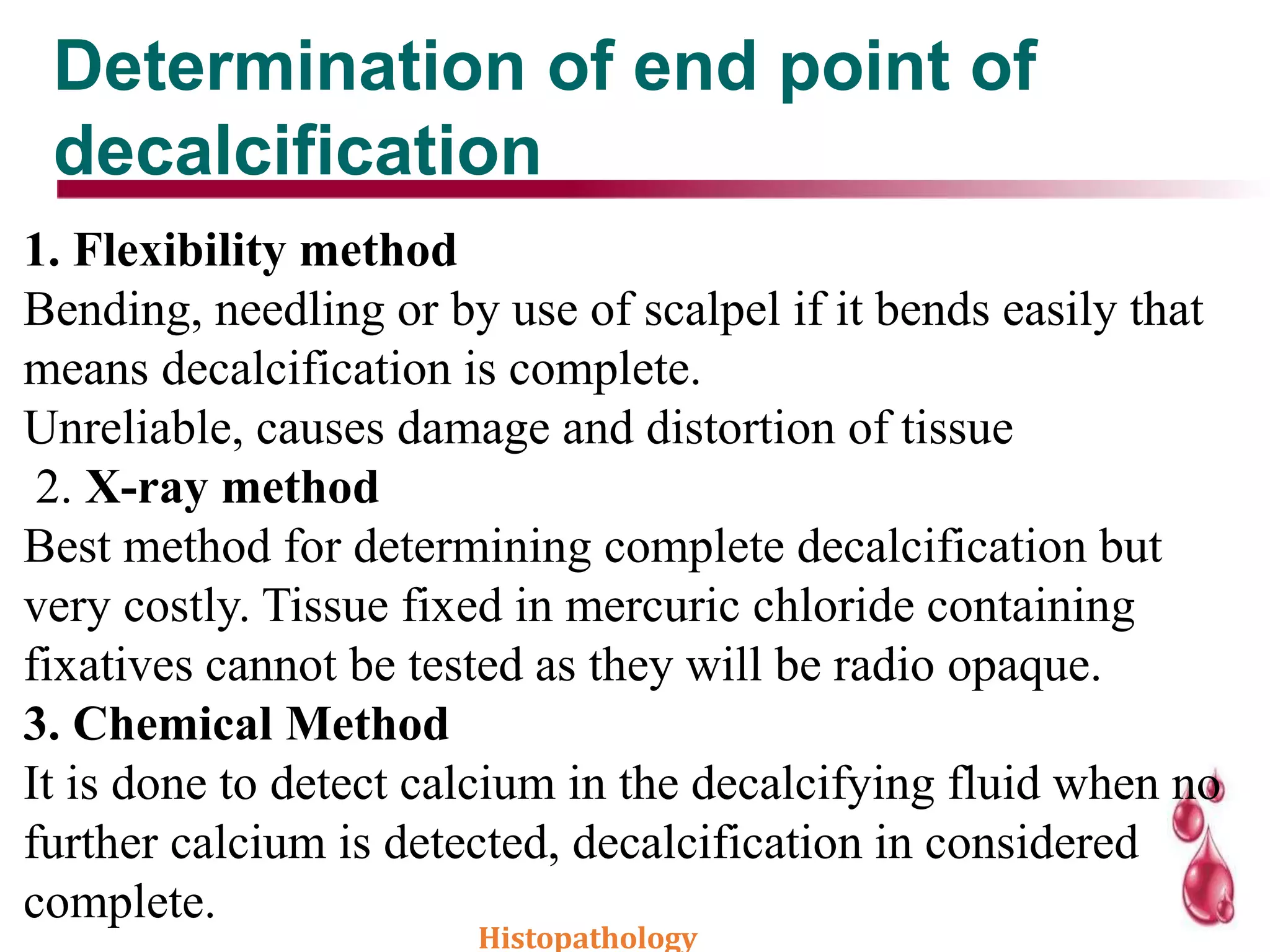
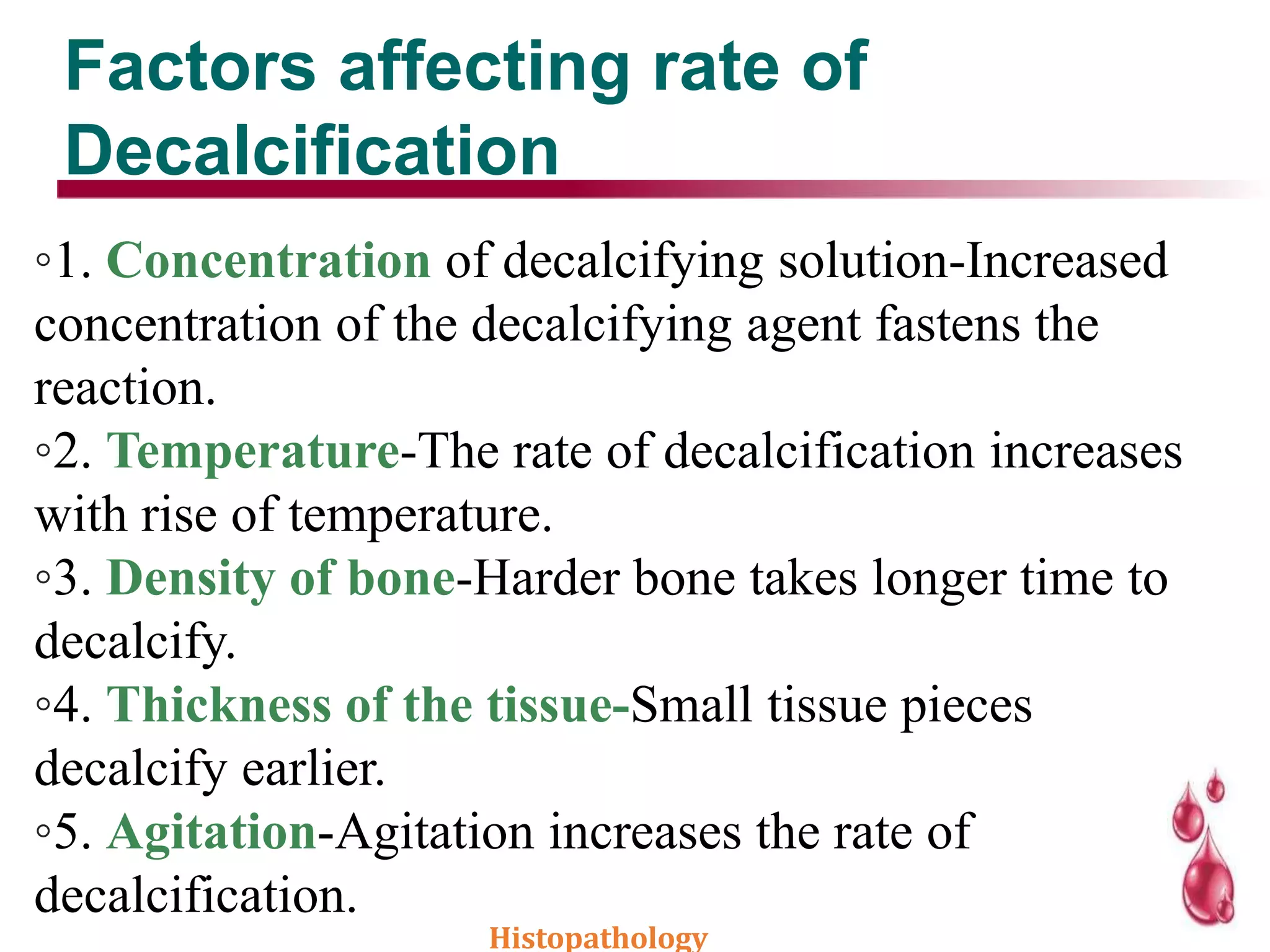
This document discusses histopathology and the process of tissue fixation. It defines histopathology as the study of diseased tissues to examine changes in structure from disease. The key steps in tissue fixation are described, including the objectives to preserve tissue structure and prevent decomposition. Various types of fixatives are classified and their mechanisms and properties explained, with examples like formalin, glutaraldehyde, alcohol, picric acid and osmium tetraoxide. Compound fixatives are also mentioned.