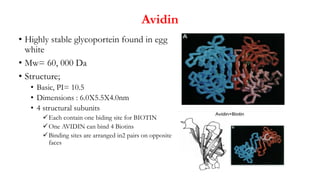
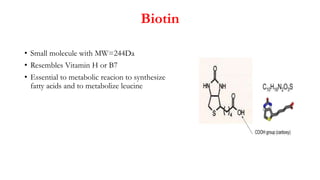
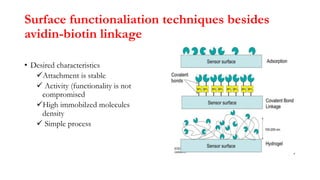
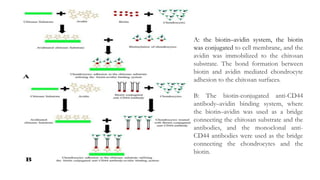
Here are the key differences between avidin, streptavidin, CaptAvidin and neutravidin:
- Avidin and streptavidin are both tetrameric proteins composed of four subunits, each capable of binding one biotin molecule.
- Streptavidin is derived from Streptomyces avidinii while avidin is derived from egg whites.
- Streptavidin is more resistant to denaturation than avidin.
- CaptAvidin is an avidin derivative where the biotin binding can be reversed by raising the pH, allowing isolation of biotinylated molecules.
- Neutravidin is a modified form of avidin that is less basic than