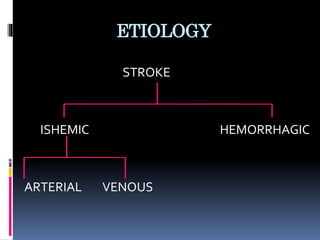
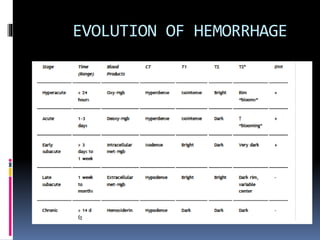
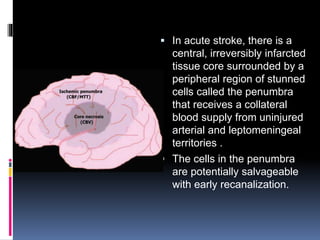
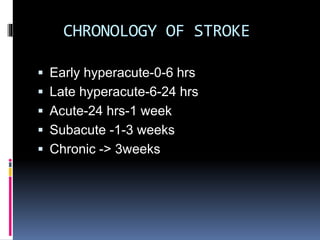
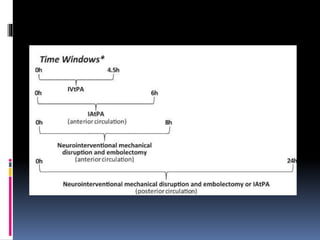
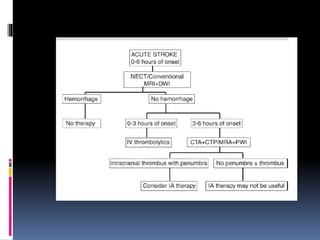
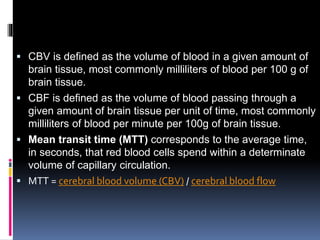
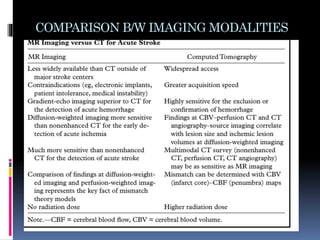
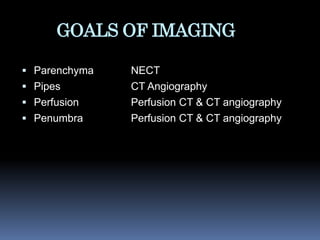
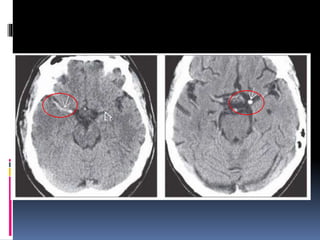
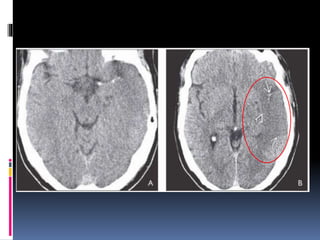
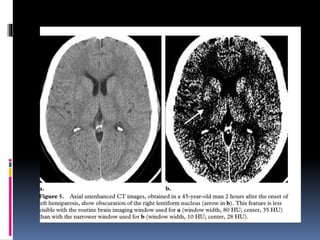
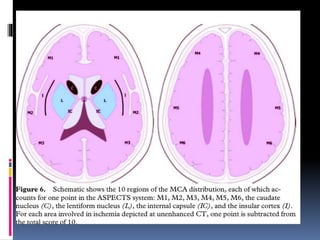
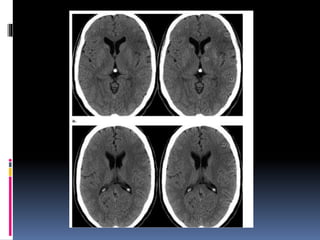
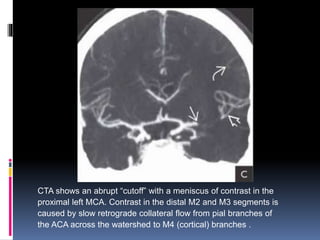
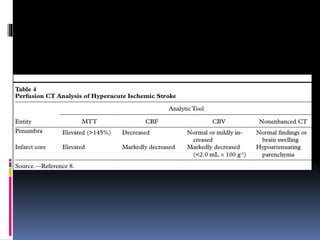
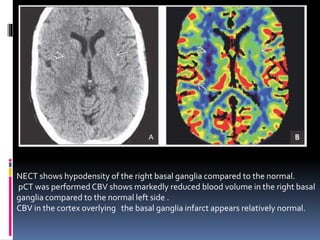
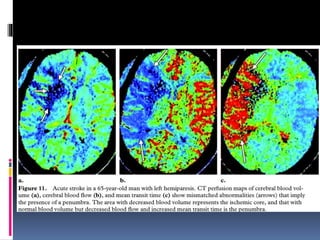
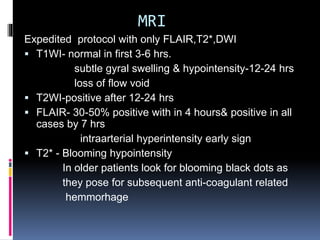
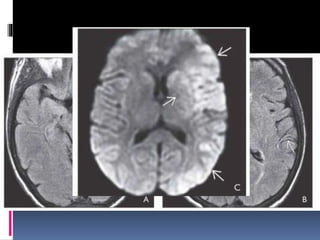
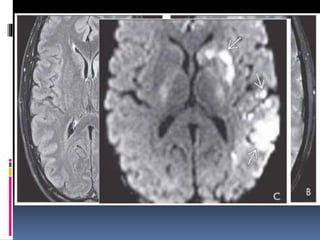
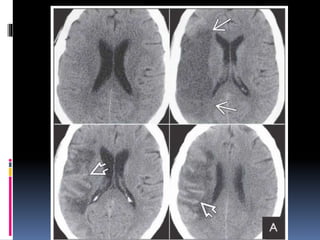
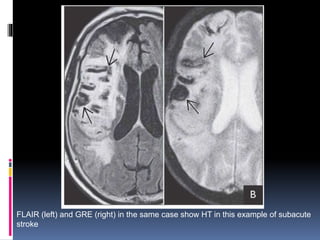
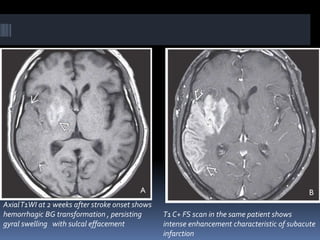
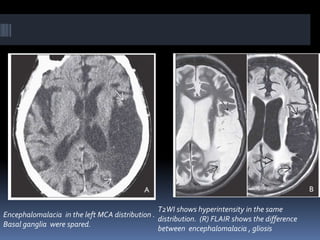
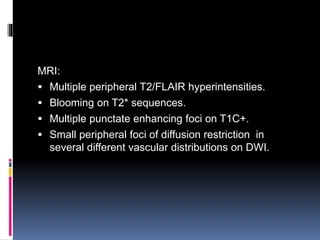
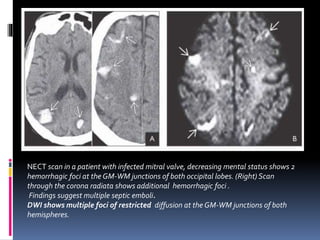
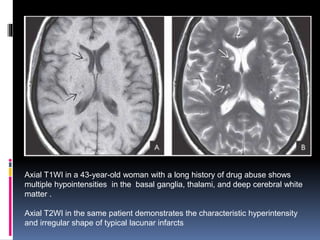
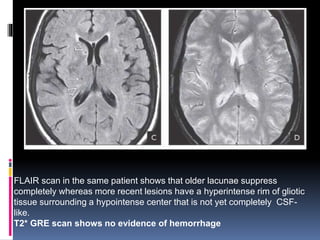
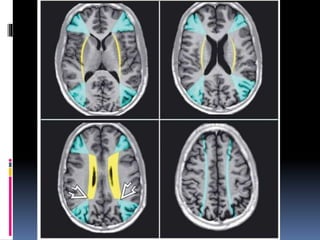
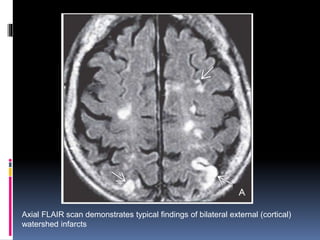
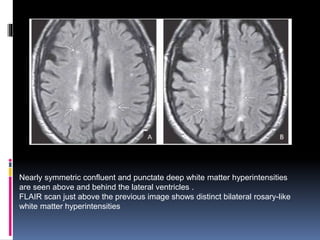
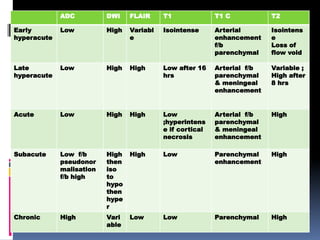
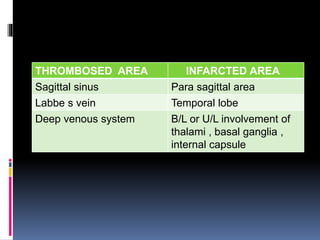
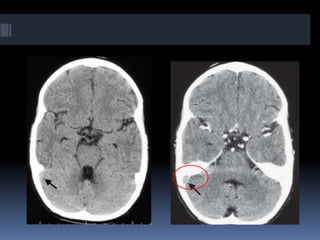
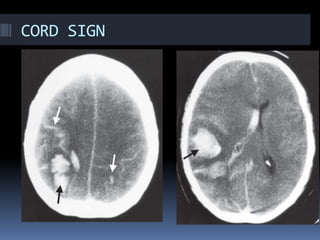
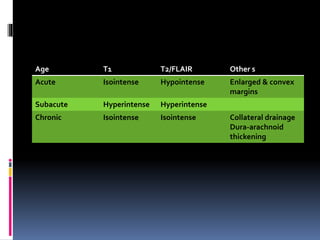
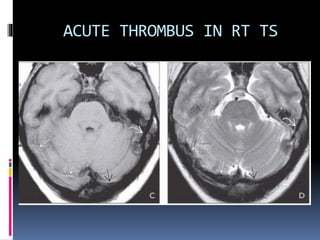
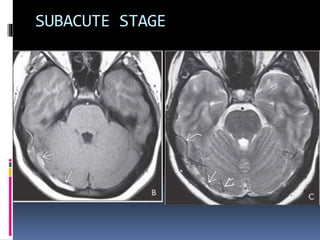
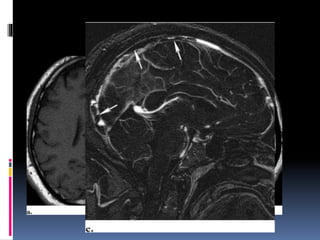
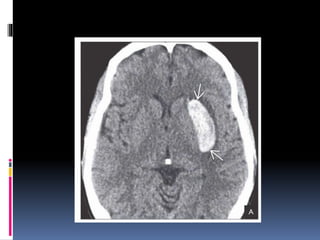
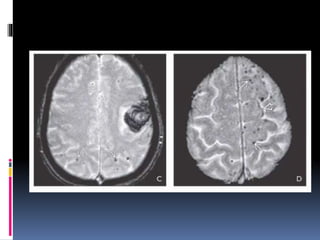
Imaging is crucial for diagnosing and guiding management of stroke. MRI is more sensitive than CT for detecting early changes, with expedited protocols using FLAIR, T2*, and DWI answering key questions acutely. Imaging can identify the type of stroke (ischemic vs. hemorrhagic), location, age of the lesion, and potential underlying causes. Understanding the imaging appearance of strokes across time points from hyperacute to chronic is important for accurate diagnosis and treatment.