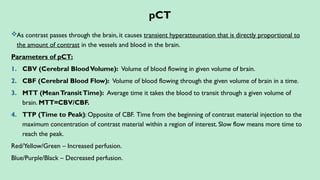
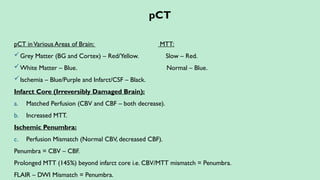
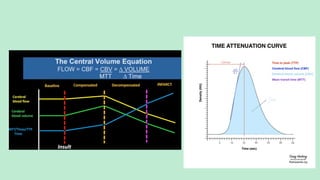
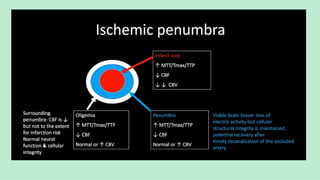
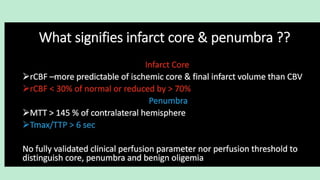
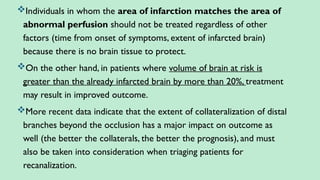
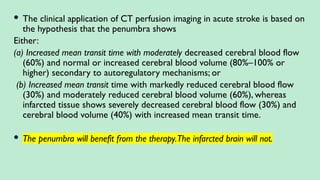
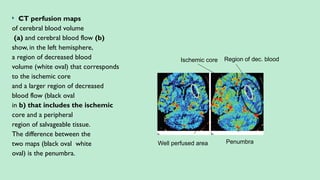
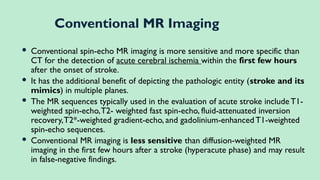
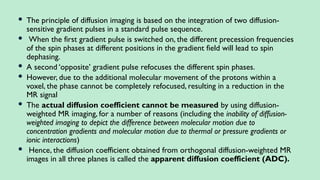
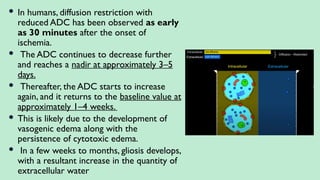
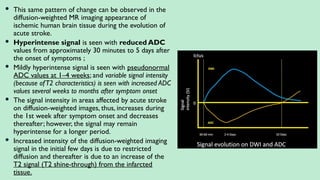
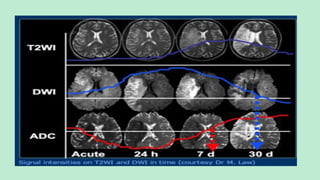
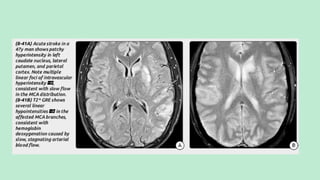
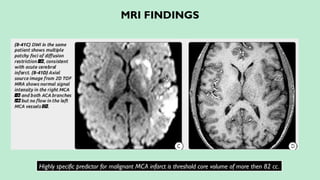
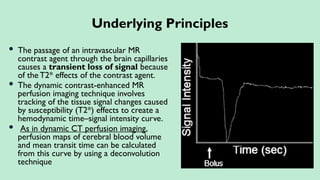
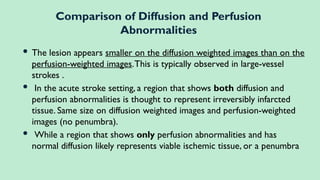
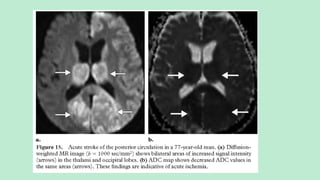
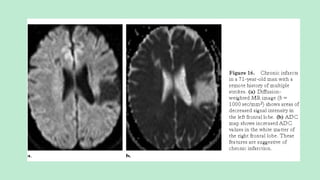
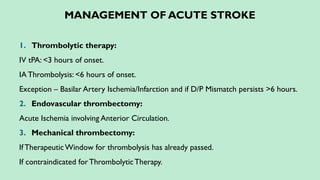
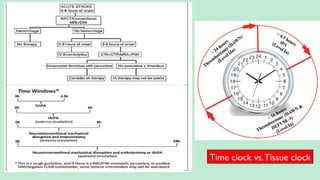
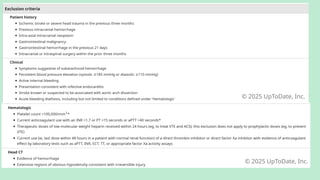
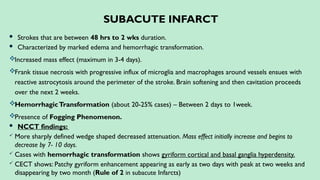
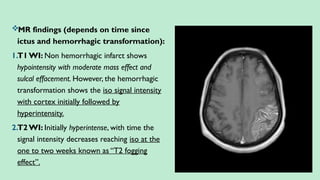
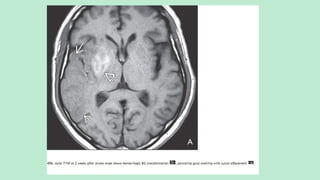
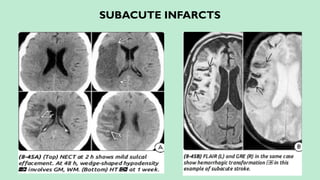
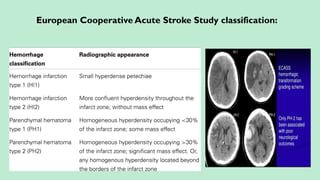
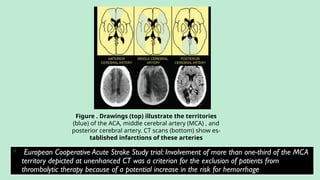
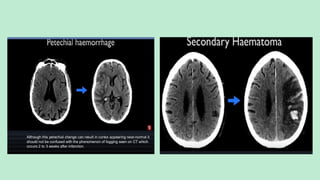
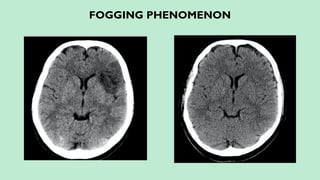
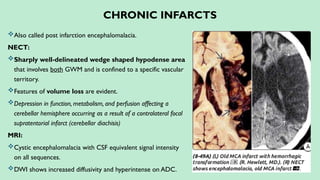
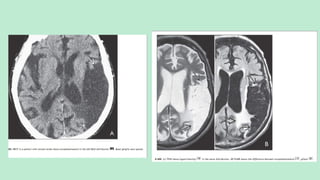
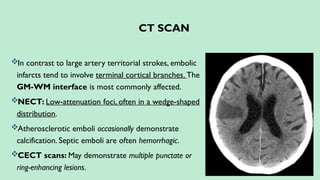
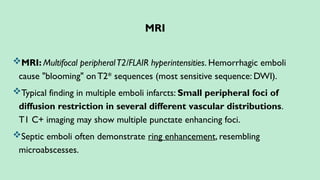
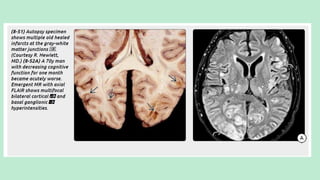
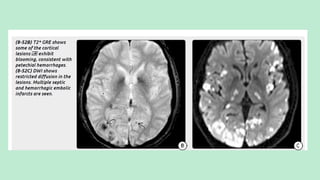
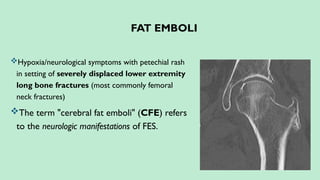
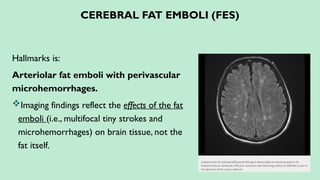
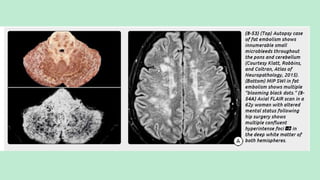
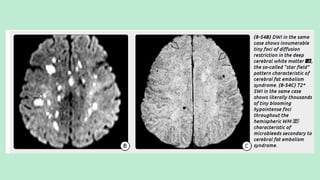
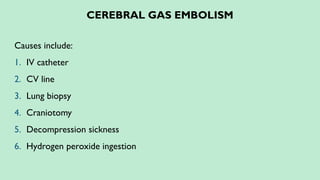
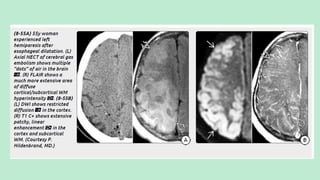
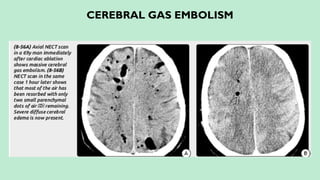
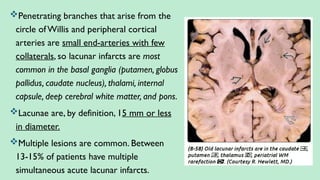
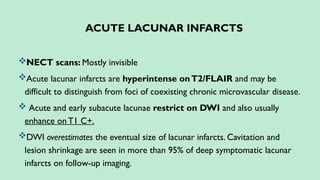
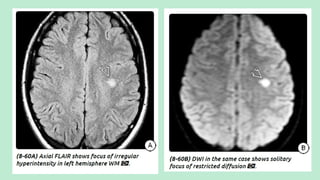
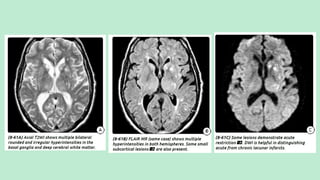
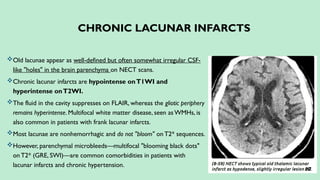
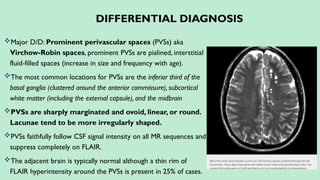
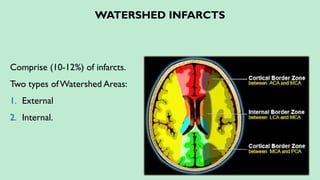
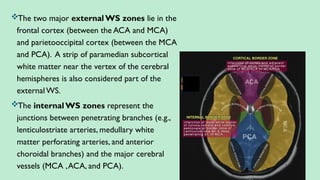
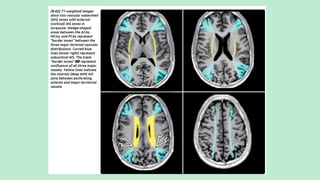
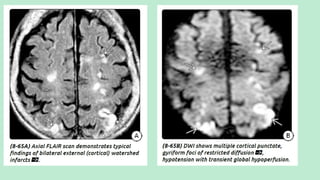
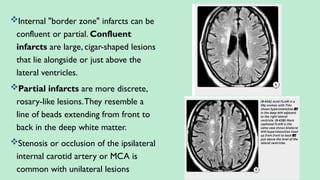
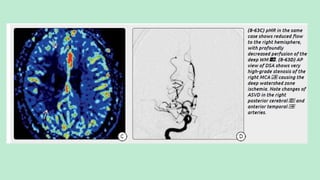
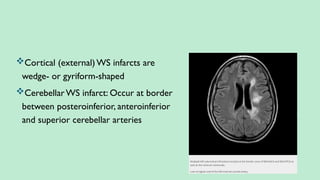
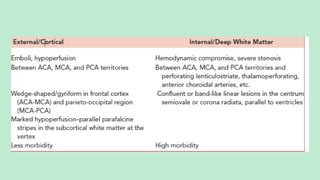
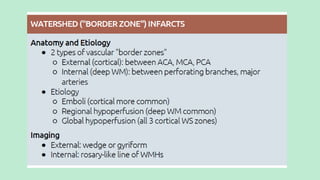
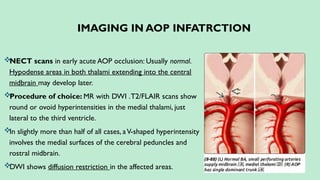
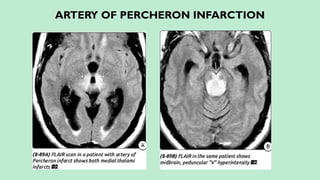
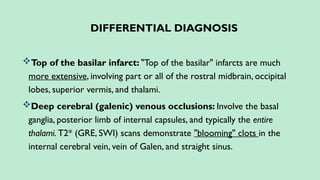
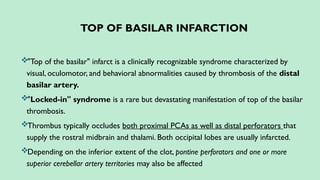
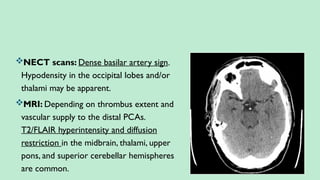
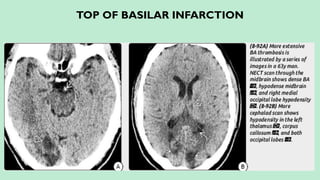
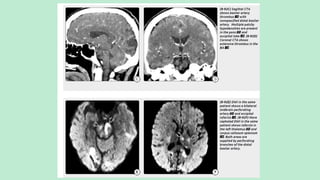
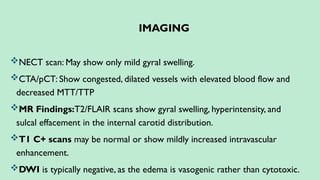
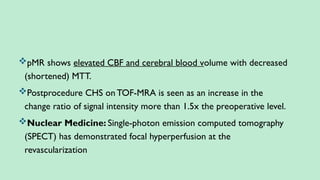
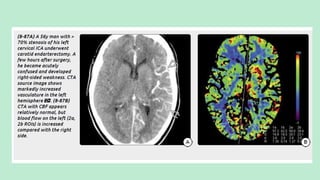
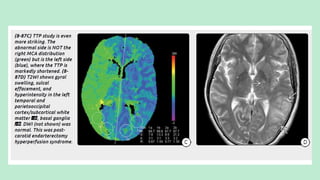
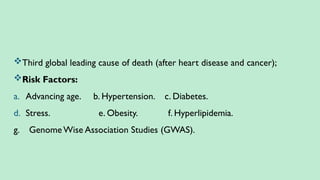
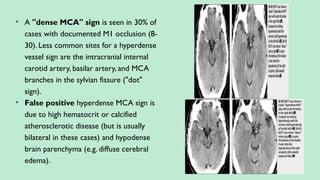
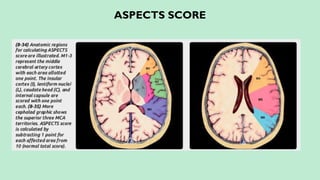
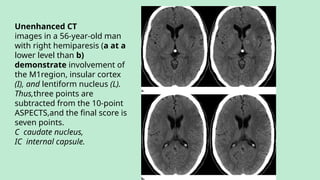
Stroke is a generic term that describes a clinical event characterized by sudden onset of a neurological deficit.
Stroke is a syndrome caused by disruption of the blood flow to part of the brain due to either:
(a) Occlusion of a blood vessel (ischemic stroke, seen in approximately 80% of cases); or
(b) Rupture of a blood vessel, resulting in injury to cells and causing sudden loss of focal brain functions. (hemorrhagic stroke).
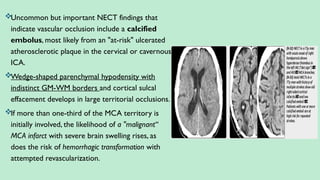
Classified into:
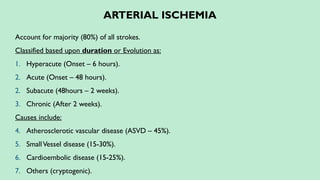
Ischemic stroke – 80%.
Hemorrhagic stroke – 20%. In this presentation we discuss everything a radiologist must know about the stroke in detail.





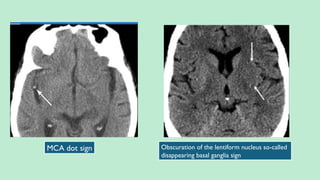
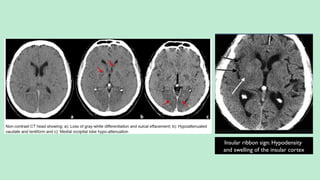


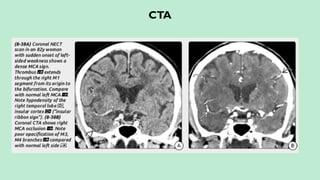
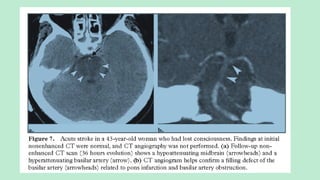
![CT ANGIOGRAPHY
Localizes and defines the extent of the intravascular thrombus.
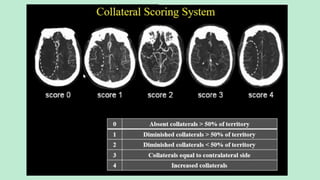
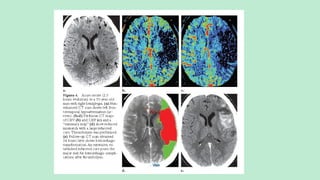
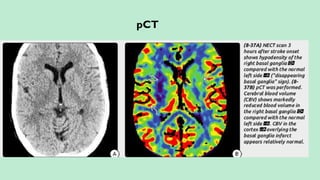
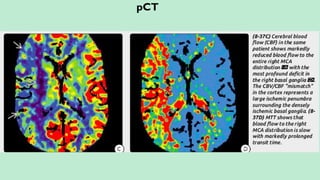
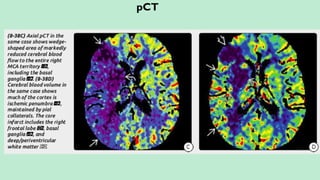
Assess collateral blood flow.
Characterizes the atherosclerotic disease.
Guidance for the interventional neuroradiologist prior to intraarterial
thrombolysis if available.
In intra-arterial thrombolysis higher chances of recanalization is seen in the
occlusion of ICA, MCA stem and basilar artery [differentiating them from
more distal (M2 or M3) occlusions for intravenous, intraarterial, or mixed
(intravenous-intraarterial) treatment planning]](https://image.slidesharecdn.com/strokeianish-250716025733-e14561b1/85/Stroke-Imaging-Fundamental-principles-and-concepts-27-320.jpg)