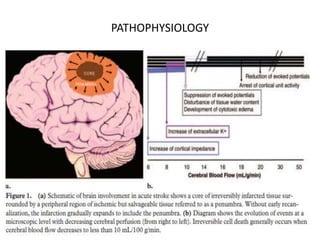
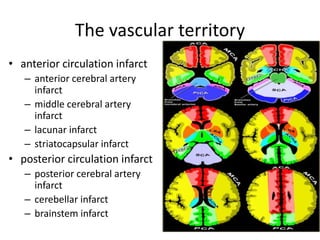
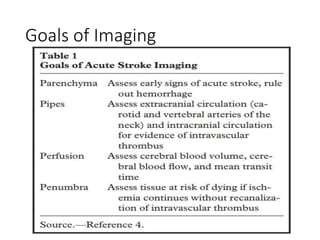
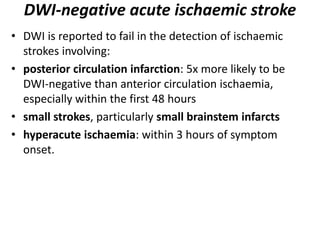
This document presents a seminar on the imaging of cerebrovascular accidents and ischemic strokes by Dr. Irko Worku, covering stroke definitions, classifications, clinical presentations, imaging techniques, and specific infarct types. It details the pathophysiology of strokes, the importance of imaging modalities like CT and MRI, and the evolution of infarction in different time frames. Additionally, it addresses complications such as cerebral venous thrombosis and cerebellar infarcts, along with the role of vascular evaluation in diagnosis and treatment.