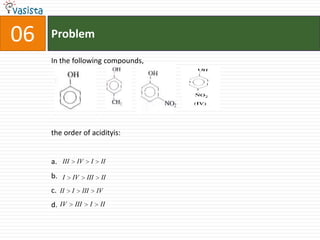
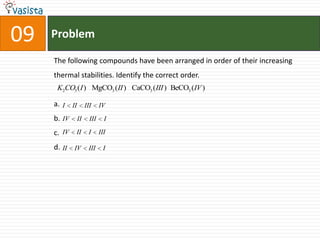
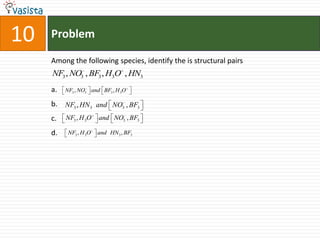
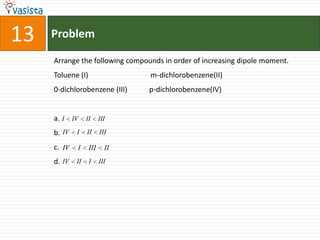
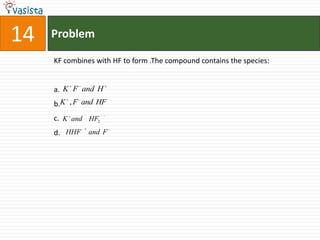
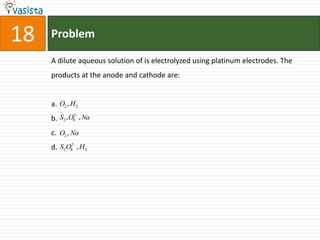
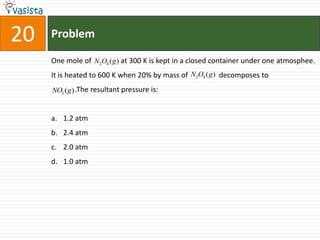
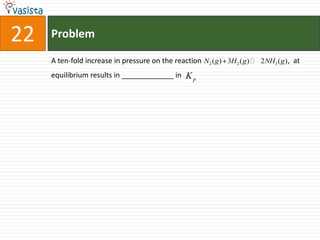
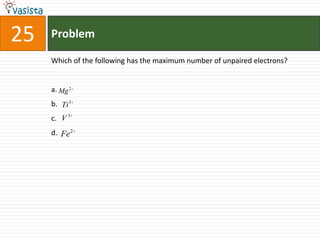
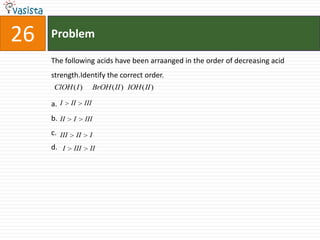
This document contains 27 chemistry problems from an unsolved 1996 IIT past paper. The problems cover a range of chemistry topics including gases, thermodynamics, kinetics, equilibrium, acids/bases, and nuclear chemistry. They involve identifying properties, relationships, and ordering compounds and reactions. The problems require calculation and reasoning skills to apply chemistry concepts.