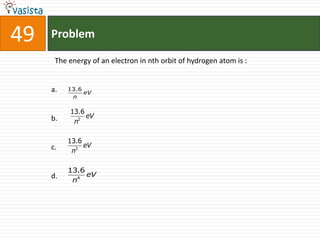
This document contains an unsolved chemistry past paper from 2002 containing 50 multiple choice questions testing various chemistry concepts. The questions cover topics such as solubility, bond energies, oxidation states, reaction types, standard reduction potentials, and thermodynamic properties. The document provides the questions along with four possible answer choices for each, but does not include the solutions.























![32 Problem
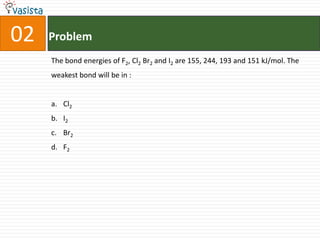
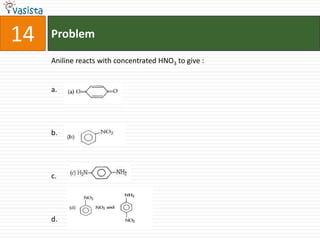
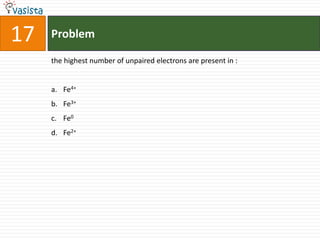
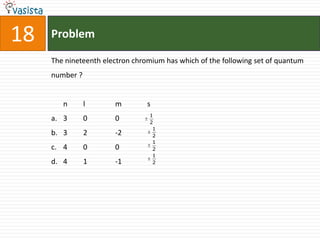
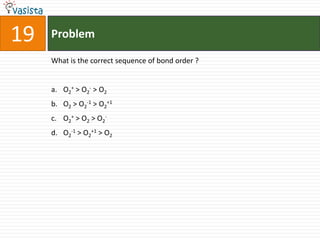
K4[Fe(CN)6] is called :
a. Prussian blue
b. Potasium ferricyanide
c. Potassium hexacyano ferrate (II)
d. Potassium hexacyanoferrate (III)](https://image.slidesharecdn.com/chemistry-2002-120103060953-phpapp02/85/AMU-Chemistry-2002-34-320.jpg)

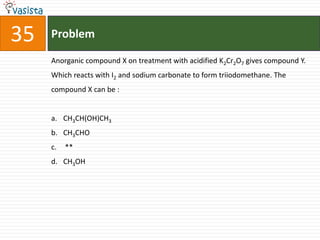






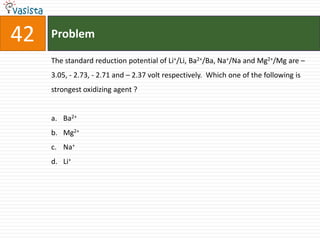

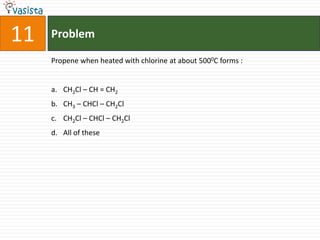
![44 Problem
Oxidation state of nitrogen is correctly given for :
Compound Oxidation state
a. Mg3N2 -3
b. NH2OH +1
c. (N2H5)2SO4 +2
d. [CO(NH3)5Cl]Cl2 0](https://image.slidesharecdn.com/chemistry-2002-120103060953-phpapp02/85/AMU-Chemistry-2002-46-320.jpg)