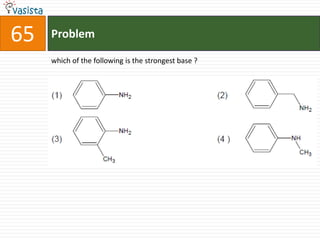
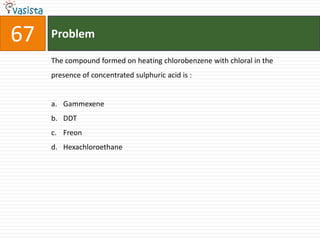
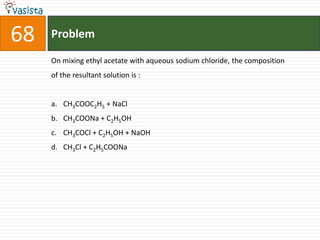
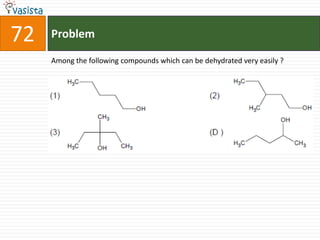
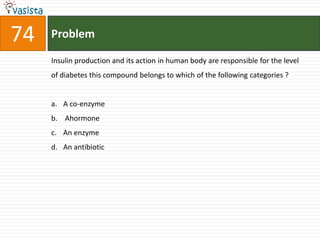
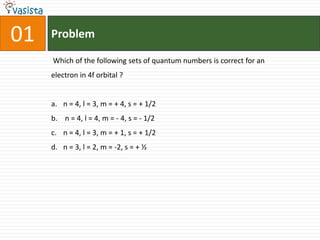
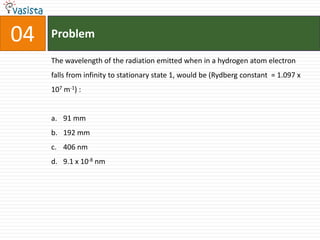
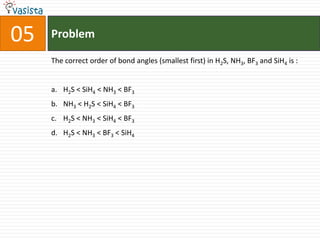
This document contains an unsolved chemistry practice test from 2004 with 50 multiple choice questions covering various topics in chemistry including quantum numbers, atomic structure, chemical bonding, acid-base reactions, solutions, equilibrium, electrochemistry, and coordination compounds. The questions require selecting the best answer from four choices given for each problem.







![Problem11Which one of the following has the regular tetrahedral structure ? XeF4SF4BF [Ni(CN)4]2-(Atomic numbers B = 5, S = 16, Ni = 28, Xe = 54)](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-13-320.jpg)





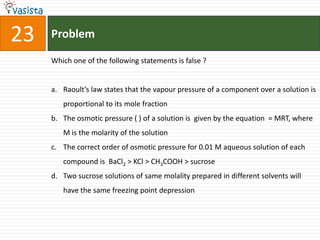




![Problem28What is the equilibrium expression for the reaction P4(s) + 5O2(g) P4O10(s) ? a.b.c. KC = [O2]5d.](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-30-320.jpg)

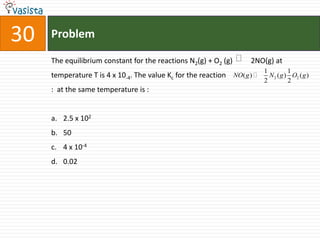
![Problem31The rate equation for the reaction 2A + B-> C is fond to be : are = k [A] [B].The correct statement in relation to this reaction is that the : Unit of k must be s-1t1/2 is a constant rate of formation of C is twice the rate of disappearance of A value of k is independent of the initial concentrations of A and B](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-33-320.jpg)

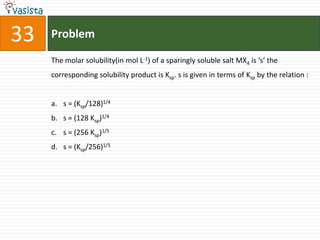
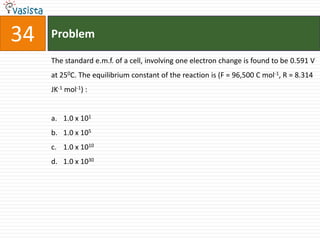





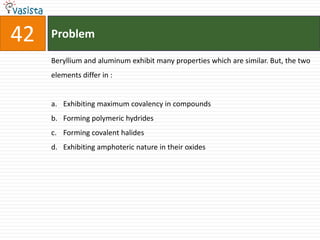
![Problem43Aluminum chloride exists as dimmer, Al2Cl6 in solid state as well as in solution of non-polar solvents such as benzene, when dissolved in water, it gives : Al3+ + 3Cl-[Al(H2O)6]3+ + 3Cl-[Al(OH)6]3- + 3HClAl2O3 + 6HCl](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-45-320.jpg)

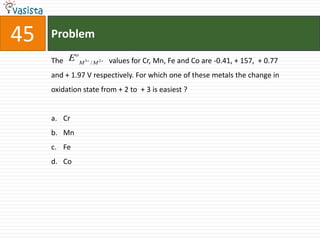

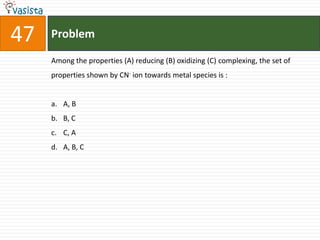

![Problem49 Which one of the following complexes is an outer orbital complex ? [Fe(CN)6]4-[Mn(CN)6]4-[Co(NH3)6]3+[Ni(NH3)6]2+(Atomic numbers Mn = 25, Fe = 36, Co = 27, Ni = 28)](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-51-320.jpg)
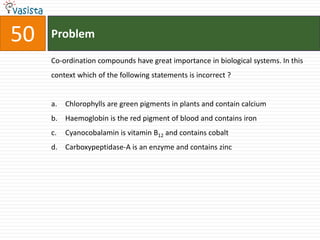

![Problem52Which one of the following has largest number of isomers ? [Ru (NH3)4 Cl2]+[Co (NH3)5Cl]2+[Ir(PR3)2 H(CO)]2+[Co(en)2Cl2]+(R = alkyl group, en = ethylenediamine)](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-54-320.jpg)
![Problem53The correct order of magnetic moments (spin only values in B.M.) among the following is [MnCl4]2- > [CoCl4]2- > [Fe(CN6)]4-[MnCl4]2-> [Fe(CN6)]4-> [CoCl4]2-Fe(CN6)]4- > [MnCl4]2- > [CoCl4]2-[Fe(CN6)]4-> [CoCl4]2-> [MnCl4]2-(Atomic numbers Mn = 25, Fe = 26, Co = 27)](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-55-320.jpg)
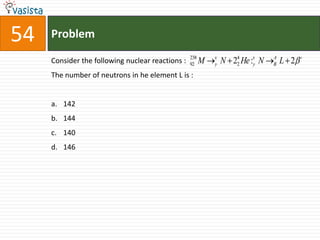

![Problem56The compound formed in the positive test for nitrogen with the Lasaigne solution of an organic compound is : Fe4[Fe(CN)6]3Na3[Fe(CN)6]Fe[Fe(CN)]3Na4[Fe(CN)6NOS]](https://image.slidesharecdn.com/chemistry-2004-110926020948-phpapp01/85/Aieee-Chemistry-2004-58-320.jpg)