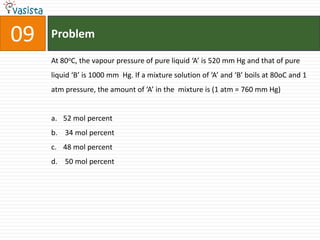
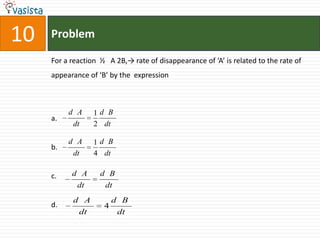
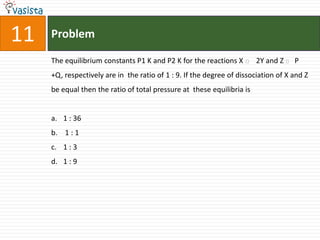
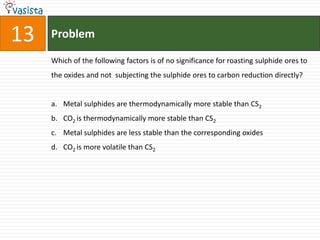
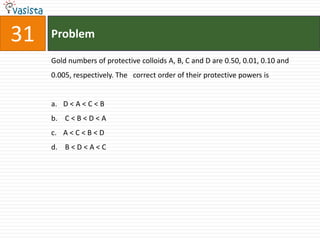
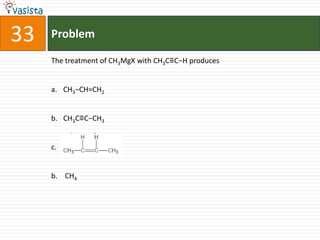
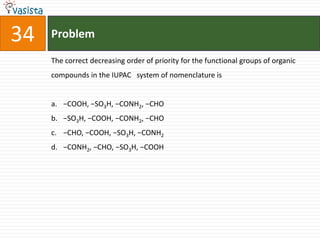
This document contains a 35 question chemistry exam with multiple choice answers. It covers topics like organic reactions, equilibrium, acid-base chemistry, and nomenclature. The questions test understanding of concepts like stereochemistry, reaction mechanisms, thermodynamics, acid strength, and IUPAC nomenclature rules. This appears to be a past exam that could be used to study for a general chemistry or organic chemistry course.



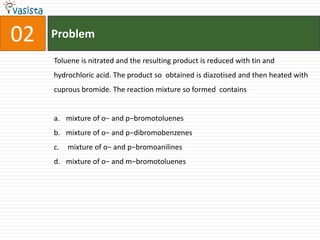
![Problem03The coordination number and the oxidation state of the element ‘E’ in the complex [E(en)2(C2O4)]NO2 (where (en) is ethylene diamine) are, respectively,6 and 2 4 and 24 and 3 6 and 3](https://image.slidesharecdn.com/aieee-chemistry-2008-110928045907-phpapp01/85/Aieee-chemistry-2008-5-320.jpg)
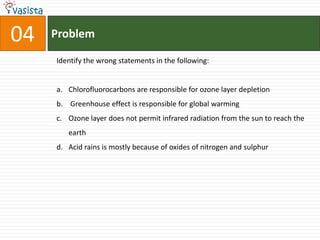
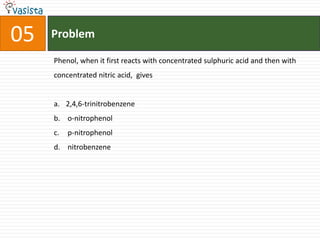
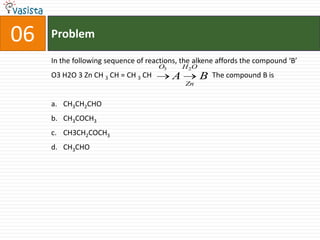

![Problem08In which of the following octahedral complexes of Co (at. no. 27), will the magnitude of Δ o be the highest?[Co(CN)6]3− [Co(C2O4)3]3− [Co(H2O)6]3+ [Co(NH3)6]3+](https://image.slidesharecdn.com/aieee-chemistry-2008-110928045907-phpapp01/85/Aieee-chemistry-2008-10-320.jpg)