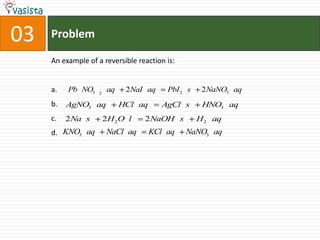
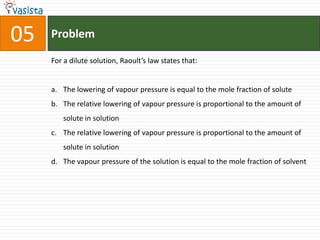
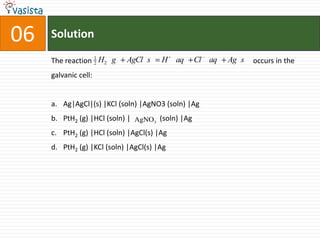
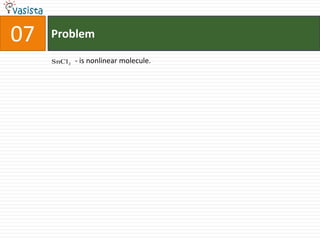
This document contains an unsolved chemistry practice paper from 1985 for the IIT JEE entrance exam. It includes multiple choice, fill-in-the-blank, true/false, and matching questions testing concepts such as the Bohr model, rates of diffusion, Raoult's law, acid-base chemistry, organic chemistry reactions, and properties of elements and compounds. The paper covers 20 sections with over 100 total questions to assess students' mastery of general chemistry topics required for the exam.