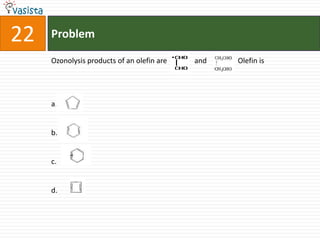
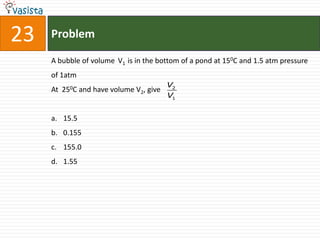
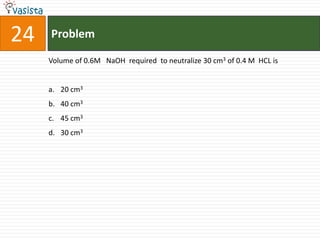
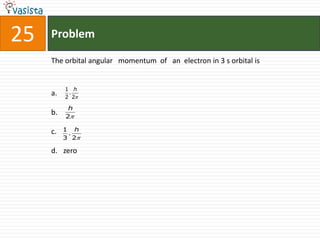
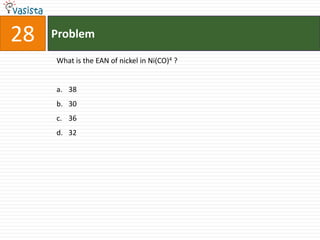
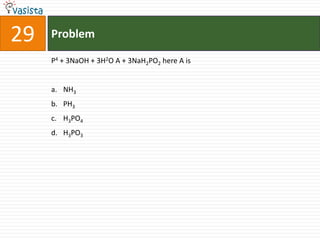
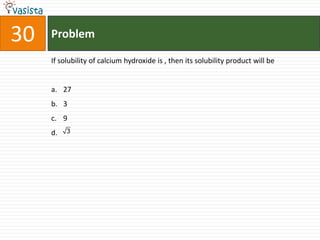
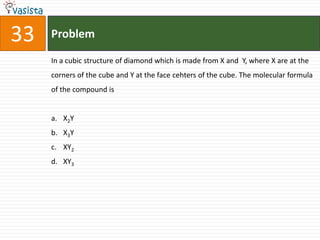
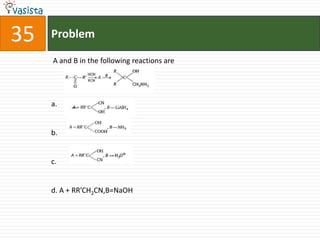
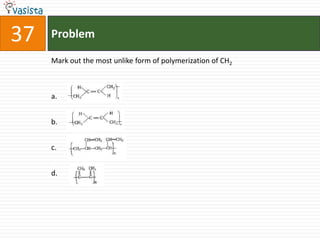
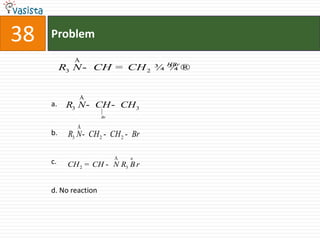
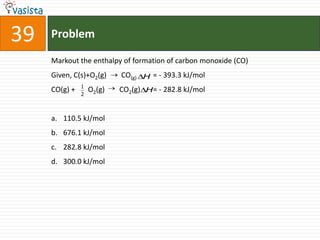
This document contains a chemistry exam from 2007 with 40 multiple choice questions covering various topics:
1. Electrochemistry questions involving standard reduction potentials.
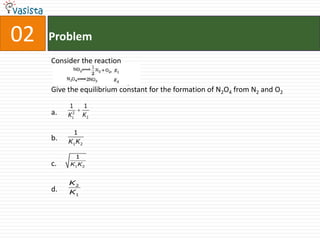
2. Equilibrium constants for reaction of N2 and O2.
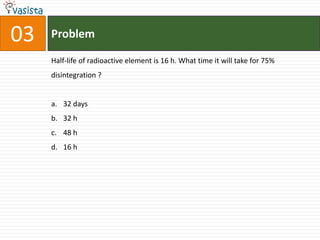
3. Radioactive decay and half-life calculations.
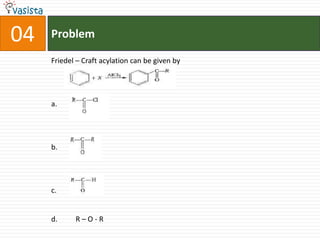
4. Organic chemistry reactions like Friedel-Crafts acylation.
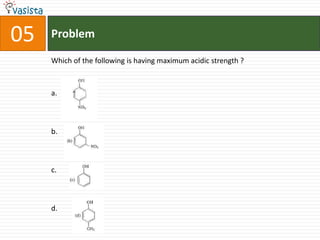
5. Properties of acids and bases.
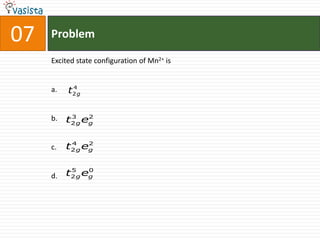
6. Characteristics of polymers, transition metals, and coordination compounds.
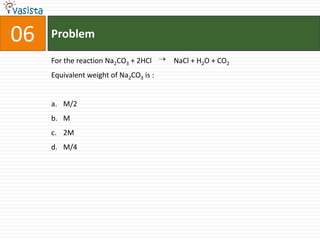
7. Stoichiometry, gas laws, thermochemistry, and other general chemistry principles.




![10 Problem
Rate of a reaction can be expressed by following rate expression
Rate=k[A]2 [B], if concentration of A is increased by 3 times, and concentration
of B increased by 2 times, how many times rate of reaction increases?
a. 9 times
b. 27 times
c. 18 times
d. 8 times](https://image.slidesharecdn.com/chemistry-2007-120103061053-phpapp01/85/AMU-Chemistry-2007-12-320.jpg)



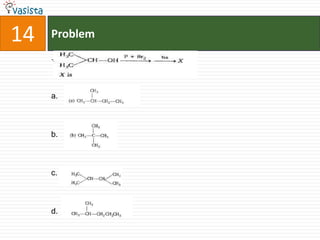

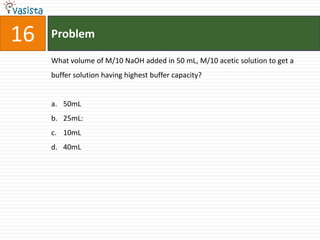


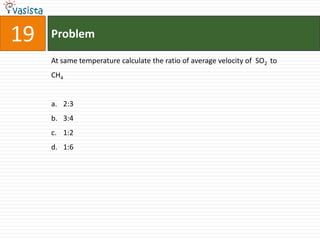
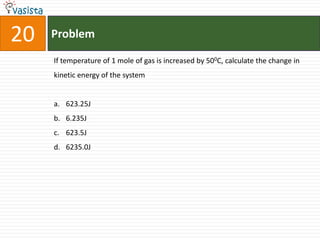
![21 Problem
Give name of the complex, name should specify the position of ligands.
a. Bistransphosphinecarbonylchloroiridium [II]
b. carbonylchlrobistransphosphineridium [III]
c. carbonylchlrobistrasphosphineridium [I]
d. chiorocarbonylbistranssphosphineriridium [I]](https://image.slidesharecdn.com/chemistry-2007-120103061053-phpapp01/85/AMU-Chemistry-2007-23-320.jpg)