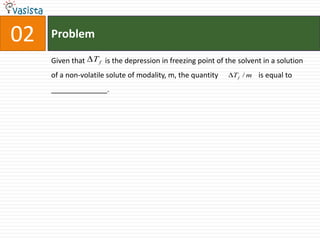
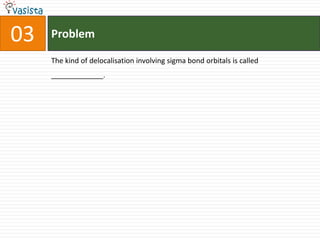
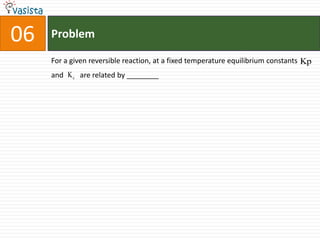
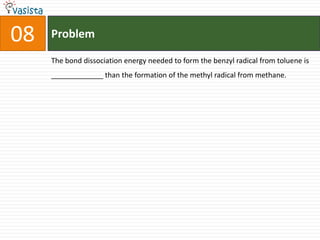
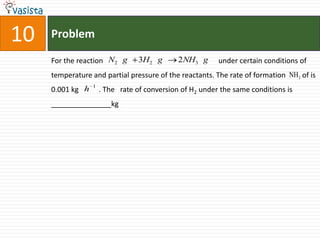
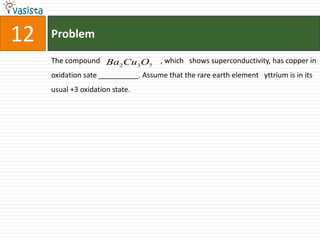
This document contains an unsolved chemistry past paper from 1994 containing 15 multiple choice or fill-in-the-blank questions. The questions cover topics such as the products of hydrolysis reactions, freezing point depression constants, types of chemical bonding including sigma bonding and delocalization, electronic configurations, types of magnetism exhibited by ions, bond dissociation energies, carbon allotropes, reaction rates, structural properties influencing solubility, IUPAC naming, and determining whether statements are true or false with explanations.