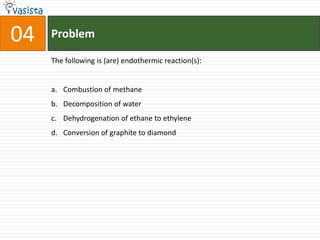
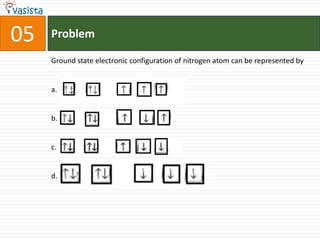
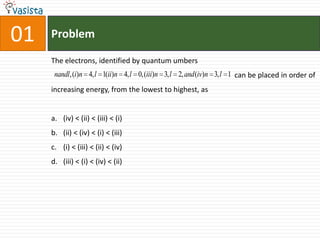
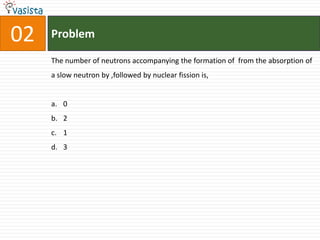
This document contains an unsolved practice paper for the IITJEE chemistry exam from 1999. It consists of 25 single answer multiple choice questions in Section I and 10 multiple answer questions in Section II covering various chemistry concepts. The questions test knowledge of topics like quantum numbers, nuclear reactions, gas laws, acid-base chemistry, coordination compounds, and organic chemistry reactions.


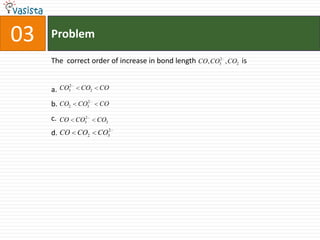


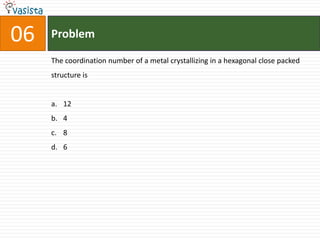


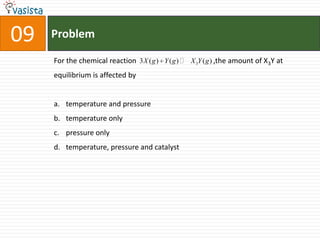
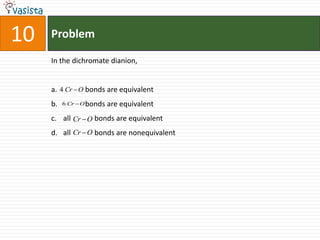

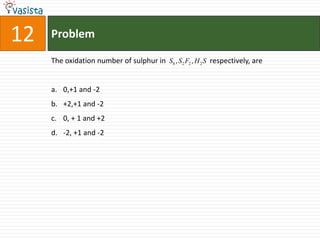

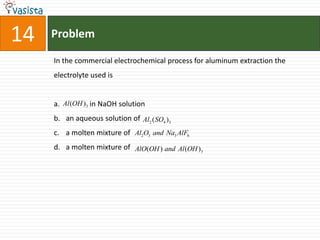



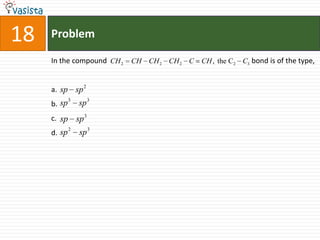

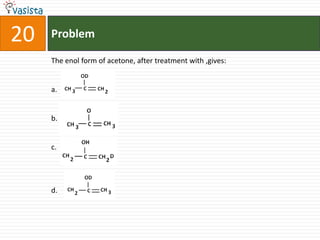

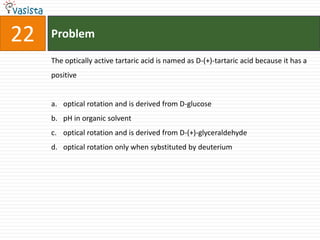
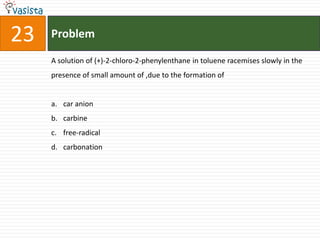

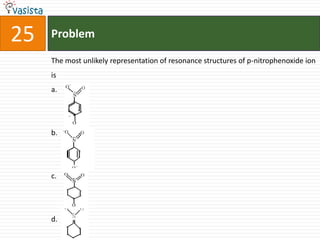

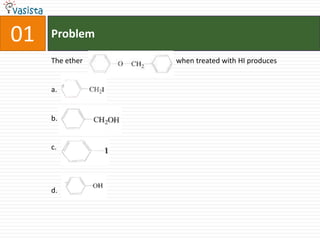

![Problem03The following statement(s) is (are) correct:A plot of log Kp versus 1/T is linearA plot of log[X]versus time is linear for a first order reaction A plot of log p versus 1/T is linear at constant volumeA plot of p vesus 1/V is linear at constant temperature](https://image.slidesharecdn.com/chemistry-1999-110923042340-phpapp02/85/IIT-JEE-Chemistry-1999-31-320.jpg)