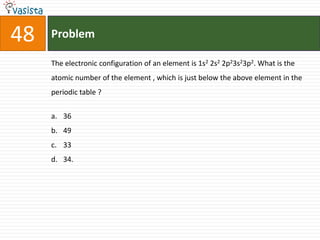
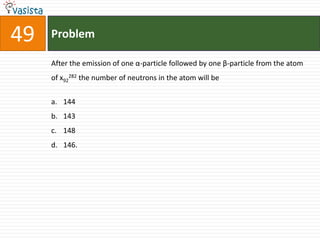
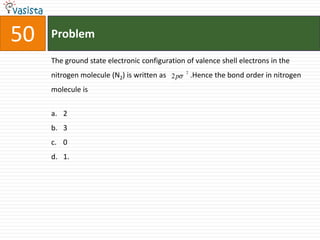
This document contains an unsolved chemistry exam from 1995 containing 50 multiple choice questions testing concepts related to general chemistry, organic chemistry, and physical chemistry. The questions cover topics such as buffers in blood, protein structure, ATP generation from glucose, enzyme properties, organic reactions, acid-base properties, radioactivity, gas laws, and thermodynamics.







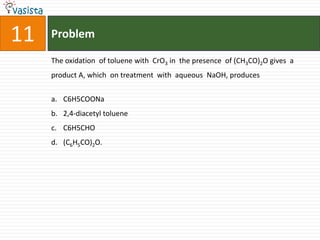

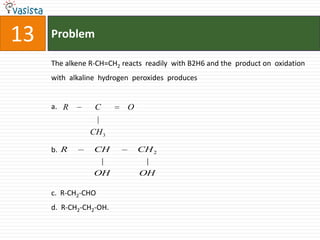

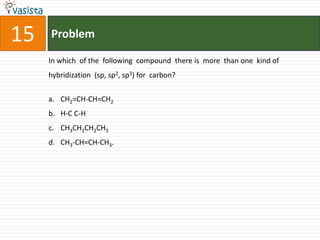

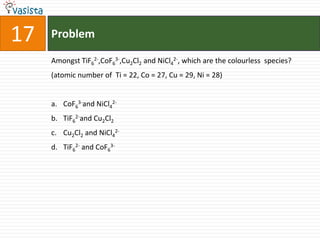
![Problem19The number of geometrical isomers for [Pt(Nh3)2Cl2], is 3142](https://image.slidesharecdn.com/chemistry1995-111012022116-phpapp01/85/AIPMT-Chemistry-1995-21-320.jpg)