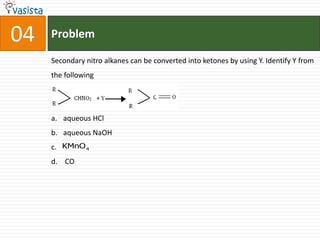
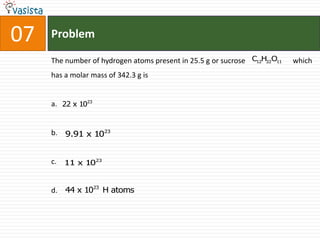
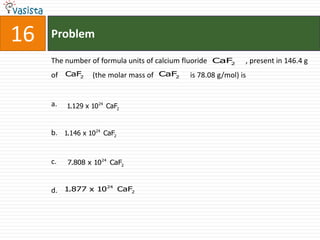
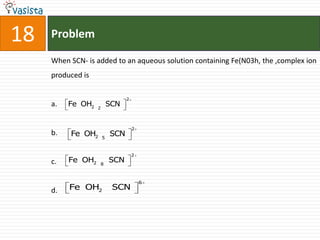
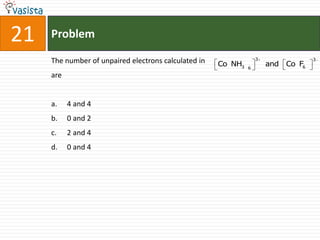
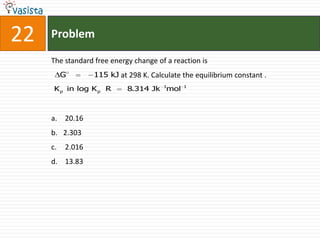
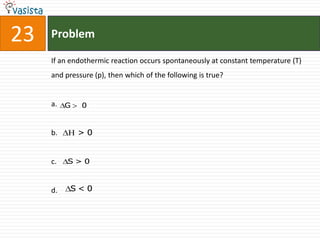
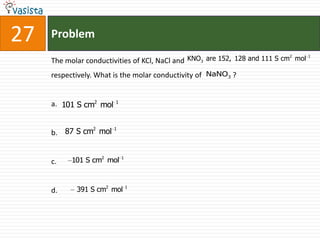
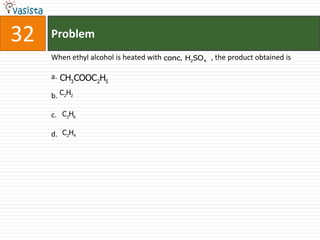
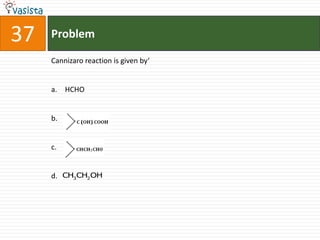
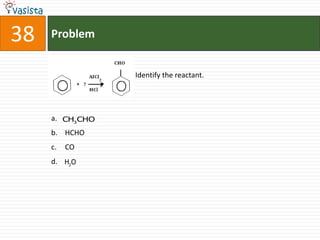
This document contains an unsolved chemistry paper from 2008 with 40 multiple choice questions testing concepts related to organic chemistry, inorganic chemistry, and physical chemistry. The questions cover topics such as reaction mechanisms, properties of compounds, oxidation states, naming of compounds, and stoichiometry.